Sodium hypochlorite
Synonym(s):bleach;Soda bleaching lye, Eau de Labarraque
- CAS NO.:7681-52-9
- Empirical Formula: ClNaO
- Molecular Weight: 74.44
- MDL number: MFCD00011120
- EINECS: 231-668-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:14:06
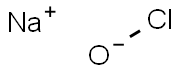
What is Sodium hypochlorite?
Description
Sodium hypochlorite (bleach) is a strong oxidizing agent. Sodium hypochlorite is unstable in solid form and therefore typically exists as a solution. Household bleach is usually about 5% sodium hypochlorite, whereas the reagent grade sodium hypochlorite sold by vendors such as Sigma Aldrich tends to be 10-15%. The pH of commercial bleach is usually 11.0 -12.5.
Chemical properties
Sodium hypochlorite (NaOCl) is a pale green crystalline solid that is unstable in air. It is soluble in cold water, with a concentration of 29.3 g/100 mL at 0°C. It decomposes in hot water and has a sweet odor. Its density is 1.6 g/cm3 and its melting point is 18°C. It decomposes in air with CO2. Its aqueous solution is very stable.
History
Chorine gas was discovered by Carl Wilhelm Scheele (1742 1786) in 1774 and known initially as depholgisticated salt spirit. In 1787, the French chemist Claude Louis Berthollet (1749 1822) experimented with aqueous solution of chlorine gas as bleaching agents. Based on Berthollet's work, the Javel Company located on the outskirts of Paris began to produce bleaches in 1788. Chlorine gas was dissolved in a solution of soda potash (potassium carbonate) to obtain a product called liqueur de Javel, which was potassium hypochlorite. Potash treated with chlorine gas was also used to produce bleaching powders. In 1820, Antoine Germaine Labarraque (1777 1850), an apothecary, substituted cheaper soda ash (sodium carbonate) for potash to produce Eau de Labarraque or Labarraque solution, which was sodium hypochlorite. Eau de Labarraque was used as a disinfectant and to bleach paper. Bleaching powders, borax, lye, and blueing were used as bleaches throughout the 19th century.
The Uses of Sodium hypochlorite

A fresh solution of sodium phosphate buffer (40 mL, pH ~6.5) consisting of a 1:1 solution of NaH2PO4 (20 mL, 0.67 M) and Na2HPO4 (20 mL, 0.67 M) was prepared. A mixture of the SM (2.1 g, 8.64 mmol), TEMPO (0.094 g, 0.604 mmol), and sodium phosphate buffer (32.2 mL, 21.59 mmol, 0.67 M) in ACN (30 mL) heated to 35 C. Solutions of NaClO2 (3.91 g, 34.5 mmol) in H2O (4.5 mL) and bleach (4.3 mL, 6% wt) were added simultaneously over 40 min. The reaction was monitored by HPLC. After 2 h, ~30% SM remained. After 6 h, ~10% SM remained. Additional bleach (100 uL) was added, and the reaction was left at RT overnight. Additional bleach (100 uL) was added. The resulting mixture was stirred at 35 C for 2 h, after which time HPLC indicated a complete conversion. The reaction mixture was quenched by the slow addition of solution of Na2SO3 (2.07 mL, 43.2 mmol) in H2O (90 mL) at 0 C, resulting in the disappearance of the brown reaction color. The solvent was removed in vacuo, and the remaining aq residue was extracted with EtOAc (3 x 40 mL). The organics were combined, washed with H2O (8 mL), brine (8 mL), dried (Na2SO4), and concentrated to provide the product as a pale yellow solid. [2.2 g, 99%]
The Uses of Sodium hypochlorite
NaOCl is a strong oxidizer used in swimming pools, and when diluted to 5.25%, it is known as the laundry bleach Clorox. Household bleach is typically a 5.25% solution, and industrial bleach is sold as a 12% solution.
What are the applications of Application
Sodium hypochlorite solution is an oxidant used with catalytic TEMPO for alcohol oxidation
Preparation
Sodium hypochlorite solution is obtained by passing chlorine into sodium hydroxide solution. The pentahydrate is obtained by crystallization.
General Description
Green to yellow watery liquid with an odor of bleaching liquid odor. Sinks and mixes with water.
Air & Water Reactions
Water soluble. Decomposes into chlorine and oxygen gases in hot water.
Reactivity Profile
Salts of hypochlorous acid, HClO. Generally toxic, irritants and powerful oxidizers, particularly in the presence of water at higher temperature as they decompose to release oxygen and chlorine gases. On contact with urea they form the highly explosive NCl3 . When heated or on contact with acids, they produce highly toxic fumes of chlorine gas [Sax, 9th ed., 1996, p. 1905]. Can react with sulfuric acid to produce heat and chlorine gas.
Hazard
Fire risk in contact with organic materials. Toxic by ingestion, strong irritant to tissue.
Health Hazard
Liquid can be irritating to skin and eyes if contact is maintained.
Fire Hazard
Behavior in Fire: May decompose, generating irritating chlorine gas.
Flammability and Explosibility
Non flammable
Side Effects
Sodium hypochlorite, commonly known as bleach, may be used as a disinfectant solution. It is a strong irritant; however, isolated reports of CoU to sodium hypochlorite exist. The mechanism for the Cou is uncertain.
Hostynek et al. describe a 36-year-old woman who developed an intensely pruritic maculopapular rash to a hypochlorite-containing cleaning product that she spilled on her leg. The rash progressed to involve her trunk and extremities and was associated with teary eyes, dyspnea, and facial edema. There was a history of a previous sensitizing event, and open testing to 1% sodium hypochlorite produced an immediate urticarial reaction. The authors suggest that this could be due to an immunological mechanism given the generalized symptoms; however, no confirmatory testing was performed and the potential of sodium hypochlorite to cause nonimmunologic Cou was evident with four of 10 controls experiencing a wheal-and-flare reaction to open application of 6% sodium hypochlorite.
Caliskan et al. described a 32-year-old female who developed severe lip edema and breathing difficulty after using a sodium hypochlorite irrigation during endodontic treatment. A scratch test to sodium hypochlorite resulted in immediate erythema and edema that began to extend up the patient’s arm. She also had a history of breathing difficulties and had developed dermatitis from her hands to elbows with the use of household cleaning agents.
Neering reported on a patient who had experienced intermittent Cou to chlorinated pools and contact with a cleansing agent containing sodium hypochlorite. A scratch test to chlorinated water was strongly positive in this patient, but negative in five controls, and closed patch testing to sodium hypochlorite was strongly positive at three hours.
Safety Profile
Mddly toxic by ingestion. Human systemic effects by ingestion: somnolence, blood pressure lowering, corrosive to skin, nausea or vomiting. Human mutation data reported. An eye irritant. Corrosive and irritating by ingestion and inhalation. The anhydrous salt is highly explosive and sensitive to heat or friction. Explosive reaction with formic acid (at So), phenylacetonitrile. Reacts to form explosive products with amines, ammonium salts (e.g., ammonium acetate, (NH4)2CO3, ammonium nitrate, ammonium oxalate, (NH4)3P04), aziridme, methanol. Violent reaction with phenyl acetonitrile, cellulose, ethyleneimine. Solutions in water are storage hazards due to oxygen evolution. When heated to decomposition it emits toxic fumes of NaaO and Cl-. Used as a bleach.
Properties of Sodium hypochlorite
| Melting point: | -16 °C |
| Boiling point: | 111 °C |
| Density | 1.25 g/mL at 20 °C |
| vapor pressure | 17.5 mmHg ( 20 °C) |
| refractive index | 1.3870 |
| storage temp. | 2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solution |
| appearance | Colorless to yellow/green liquid |
| Specific Gravity | 1.209 |
| color | Light yellow |
| Odor | pale greenish to yel. liq., chlorine bleach odor |
| Water Solubility | decomposes. |
| Merck | 14,8628 |
| Exposure limits | ACGIH: Ceiling 2 mg/m3 OSHA: TWA 2 mg/m3 NIOSH: IDLH 10 mg/m3; Ceiling 2 mg/m3 |
| Dielectric constant | 6.7(Ambient) |
| Stability: | Stable. Contact with acids releases poisonous gas ( chlorine ). Light sensitive. Incompatible with strong acids, amines, ammonia, ammonium salts, reducing agents, metals, aziridine, methanol, formic acid, phenylacetonitrile. |
| CAS DataBase Reference | 7681-52-9(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium hypochlorite (7681-52-9) |
Safety information for Sodium hypochlorite
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium hypochlorite
Sodium hypochlorite manufacturer
JSK Chemicals
Raj Biosis Private Limited
ASM Organics
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Sodium Hypochlorite 99%View Details
Sodium Hypochlorite 99%View Details -
 SODIUM HYPO CHLORITE 98%View Details
SODIUM HYPO CHLORITE 98%View Details -
 Sodium hypochlorite 99%View Details
Sodium hypochlorite 99%View Details -
 Sodium Hypochlorite Solution CAS 7681-52-9View Details
Sodium Hypochlorite Solution CAS 7681-52-9View Details
7681-52-9 -
 Sodium Hypochlorite CAS 7681-52-9View Details
Sodium Hypochlorite CAS 7681-52-9View Details
7681-52-9 -
 Sodium Hypochlorite Solution, 4-6% CAS 7681-52-9View Details
Sodium Hypochlorite Solution, 4-6% CAS 7681-52-9View Details
7681-52-9 -
 Liquid Sodium HypochloriteView Details
Liquid Sodium HypochloriteView Details
14380-61-1 -
 Sodium Hypochlorite Liquid BleachView Details
Sodium Hypochlorite Liquid BleachView Details
7681-52-9
