Sodium chloride
Synonym(s):NaCl;Sodium chloride;NaCl 10 Tablets;Natrii chloridum;Physiological Saline, tablets
- CAS NO.:7647-14-5
- Empirical Formula: ClNa
- Molecular Weight: 58.44
- MDL number: MFCD00003477
- EINECS: 231-598-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:18:42
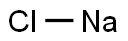
What is Sodium chloride?
Absorption
Absorption of sodium in the small intestine plays an important role in the absorption of chloride, amino acids, glucose, and water. Chloride, in the form of hydrochloric acid (HCl), is also an important component of gastric juice, which aids the digestion and absorption of many nutrients.
Toxicity
The rare inadvertent intravascular administration or rapid intravascular absorption of hypertonic sodium chloride can cause a shift of tissue fluids into the vascular bed, resulting in hypervolemia, electrolyte disturbances, circulatory failure, pulmonary embolism, or augmented hypertension.
Description
Sodium chloride is widely distributed in nature, with oceans being its primary source at an average concentration of 2.68 wt% in seawater. It also occurs in inland saline waters and sedimentary salt deposits such as halite. As the most significant salt of sodium and chlorine, it is essential in food preparation for flavoring, nutritional sodium supply, and preservation. Therapeutically, NaCl solutions are used as electrolyte replenishers to prevent dehydration and as emetics. In the chemical industry, it serves as the starting material for producing key compounds including hydrochloric acid, sodium hydroxide, sodium carbonate, and metallic sodium, and is also employed in textile dyeing, pottery glazing, soap manufacturing, leather curing, and freezing mixtures.
Chemical properties
Sodium chloride (NaCl), commonly known as table salt or halite, is a white crystalline solid soluble in water, slightly soluble in alcohol, and with a melting point of 804°C (1480°F). As the most important sodium mineral, it occurs naturally in seawater, underground deposits, and brine wells. It serves as a fundamental raw material for producing chlorine, sodium hypochlorite, sodium bisulfate, soda ash, and hydrogen chloride, and is widely used in food processing, fertilizer manufacturing, and de-icing of roads.
Physical properties
Sodium chloride (NaCl), commonly known as table salt, is an ionic compound that forms white cubic crystals with alternating sodium and chloride ions. Its mineral form, halite, is found worldwide in natural deposits and constitutes approximately 2.7% by weight of dissolved minerals in seawater. Essential for life, the average adult requires 1 to 2 grams daily to maintain cellular water balance, nerve signal transmission, and muscle contraction.
Occurrence
Sodium chloride is widely distributed in nature, with oceans being its primary source at an average concentration of 2.68% in seawater. It also occurs in inland saline waters and sedimentary salt deposits such as halite. As the most important salt of sodium and chlorine, it is essential in food preparation for flavoring, nutritional sodium supply, and preservation. Therapeutically, NaCl solutions are used as electrolyte replenishers to prevent dehydration and as emetics. In the chemical industry, it serves as the starting material for producing key compounds including hydrochloric acid, sodium hydroxide, sodium carbonate, and metallic sodium, and is also employed in textile dyeing, pottery glazing, soap manufacturing, leather curing, and freezing mixtures.
The Uses of Sodium chloride
Sodium chloride (NaCl) is used in biochemistry and molecular biology as a component of PBS and SSC buffers. Its saturated aqueous solution, known as brine, is commonly employed to remove water from organic solvents after extractions. Additionally, NaCl functions as a preservative, astringent, and antiseptic for treating inflamed lesions, and can mask odors, reduce product density, and control viscosity. Diluted solutions are non-irritating.
Indications
This intravenous solution is indicated for use in adults and pediatric patients as a source of electrolytes and water for hydration. Also, designed for use as a diluent and delivery system for intermittent intravenous administration of compatible drug additives.
What are the applications of Application
NaCl Solution, 1M is stock solution used as a buffer in a wide variety
Background
Sea salt is the salt obtained from the evaporation of seawater or water from saltwater lakes. Sea salt production is subject to little processing which leaves certain trace minerals and elements behind. In comparison, table salt is mined from underground sedimentary deposits and is more heavily processed to eliminate minerals. Unlike sea salt, table salt usually contains an additive to prevent clumping and involves addition of iodine. Sea salt is a food ingredient and is often colored by adding charcoal or red clay to sometimes be referred to as “Hawaiian Sea Salt.”
Definition
ChEBI: An inorganic chloride salt having sodium(1+) as the counterion.
Preparation
Sodium chloride is produced by solar evaporation of seawater or brine from underground salt deposits. It also is produced by mining rock salt. The commercial product contains small amounts of calcium and magnesium chlorides.
Production Methods
Sodium chloride occurs naturally as the mineral halite. Commercially, it is obtained by the solar evaporation of sea water, by mining, or by the evaporation of brine from underground salt deposits.
Definition
A compound with an acidic and a basic radical, or a compound formed by total or partial replacement of the hydrogen in an acid by a metal. In general terms a salt is a material that has identifiable cationic and anionic components.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sodium chloride is generally unreactive. Releases gaseous hydrogen chloride if mixed with a concentrated nonvolatile acid such as sulfuric acid.
Fire Hazard
Literature sources indicate that Sodium chloride is nonflammable.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Sodium chloride is widely used in various parenteral and nonparenteral pharmaceutical formulations, primarily to prepare isotonic solutions. While historically employed as a lubricant and diluent in capsules and direct-compression tablets—a practice now less common—it continues to serve as a channeling agent and osmotic agent in controlled-release tablet cores, as well as a porosity modifier in tablet coatings and a controller of drug release from microcapsules. Its addition to aqueous spray-coating solutions containing hydroxypropyl cellulose or hypromellose suppresses the agglomeration of crystalline cellulose particles, and it can modulate drug release from gels and emulsions, control micelle size, and adjust the viscosity of polymer dispersions by altering the ionic character of formulations.
Biochem/physiol Actions
Sodium chloride helps to stimulate the stable induction of T-helper cell 17 (TH17) cells.
Clinical Use
Treatment and prophylaxis of sodium chloride deficiency
Safety Profile
Poison by intraperitoneal and intracervical routes. Moderately toxic by ingestion, intravenous, and subcutaneous routes. An experimental teratogen. Human systemic effects by ingestion: blood pressure increase. Human reproductive effects by intraplacental route: terminates pregnancy. Experimental reproductive effects. Human mutation data reported. A skin and eye irritant. When bulk sodium chloride is heated to high temperature, a vapor is emitted that is irritating, particularly to the eyes. Ingestion of large amounts of sodium chloride can cause irritation of the stomach. Improper use of salt tablets may produce this effect. Potentially explosive reaction with dichloromaleic anhydride + urea. Electrolysis of mixtures with nitrogen compounds may form the explosive nitrogen trichloride. Reaction with burning lithmm forms the dangerously reactive sodmm. The molten salt at 11 00' reacts explosively with water. Violent reaction with BrF3. When heated to decomposition it emits toxic fumes of Cland Na2O.
Safety
Sodium chloride is the most important salt in the body for
maintaining the osmotic tension of blood and tissues. About
5–12 g of sodium chloride is consumed daily, in the normal adult
diet, and a corresponding amount is excreted in the urine. As an
excipient, sodium chloride may be regarded as an essentially
nontoxic and nonirritant material. However, toxic effects following
the oral ingestion of 0.5–1.0 g/kg body-weight in adults may occur.
The oral ingestion of larger quantities of sodium chloride, e.g.
1000 g in 600mL of water, is harmful and can induce irritation
of the gastrointestinal tract, vomiting, hypernatremia, respiratory
distress, convulsions, or death.
In rats, the minimum lethal intravenous dose is 2.5 g/kg bodyweight.
LD50 (mouse, IP): 6.61 g/kg
LD50 (mouse, IV): 0.65 g/kg
LD50 (mouse, oral): 4.0 g/kg
LD50 (mouse, SC): 3.0 g/kg
LD50 (rat, oral): 3.0 g/kg
Drug interactions
Potentially hazardous interactions with other drugs
May impair the efficacy of antihypertensive drugs in
chronic renal failure.
Biological function
Sodium, the major cation of the extracellular fluid, functions primarily in controlling body fluids' water distribution, fluid balance, and osmotic pressure. Sodium is also associated with chloride and bicarbonate in the regulation of the acid-base equilibrium of body fluid. Chloride, the major extracellular anion, closely follows the metabolism of sodium, and changes in the acid-base balance of the body are reflected by changes in the chloride concentration. Sodium chloride (NaCl), also known as salt, is an essential compound the body uses to absorb and transport nutrients, maintain blood pressure, maintain the right balance of fluid, transmit nerve signals, and contract and relax muscles.
Metabolism
The salt that is taken in to gastro intestinal tract remains for the most part unabsorbed as the liquid contents pass through the stomach and small bowel. On reaching the colon this salt, together with the water is taken in to the blood. As excesses are absorbed the kidney is constantly excreting sodium chloride, so that the chloride level in the blood and tissues remains fairly constant.Further more, if the chloride intake ceases, the kidney ceases to excrete chlorides. Body maintains an equilibrium retaining the 300gm of salt dissolved in the blood and fluid elements of the tissue dissociated into sodium ions and chloride ions.
Metabolism
Excess sodium is mainly excreted by the kidney, and small amounts are lost in the faeces and sweat.
Storage
Aqueous sodium chloride solutions are stable but may cause the
separation of glass particles from certain types of glass containers.
Aqueous solutions may be sterilized by autoclaving or filtration.
The solid material is stable and should be stored in a well-closed
container, in a cool, dry place.
It has been shown that the compaction characteristics and the
mechanical properties of tablets are influenced by the relative
humidity of the storage conditions under which sodium chloride
was kept.
Purification Methods
It is recrystallised from a saturated aqueous solution (2.7mL/g) by passing in HCl gas, or by adding EtOH or acetone. It can be freed from bromide and iodide impurities by adding chlorine water to an aqueous solution and boiling it for some time to expel free bromine and iodine. Traces of iron can be removed by prolonged boiling of solid NaCl in 6M HCl; the crystals are then washed with EtOH and dried at ca 100o. Sodium chloride has been purified by sublimation in a stream of pre-purified N2 and collected by electrostatic discharge [Ross & Winkler J Am Chem Soc 76 2637 1954]. For use as a primary analytical standard, analytical reagent grade NaCl should be finely ground, dried in an electric furnace at 500-600o in a platinum crucible, and allowed to cool in a desiccator. For most purposes, however, drying at 110-120o is satisfactory.
Incompatibilities
Aqueous sodium chloride solutions are corrosive to iron. They also react to form precipitates with silver, lead, and mercury salts. Strong oxidizing agents liberate chlorine from acidified solutions of sodium chloride. The solubility of the antimicrobial preservative methylparaben is decreased in aqueous sodium chloride solutions and the viscosity of carbomer gels and solutions of hydroxyethyl cellulose or hydroxypropyl cellulose is reduced by the addition of sodium chloride.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (injections; inhalations; nasal, ophthalmic, oral, otic, rectal, and topical preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Sodium chloride
| Melting point: | 801 °C (lit.) |
| Boiling point: | 1465 °C/1 atm (lit.) |
| Density | 1.199 g/mL at 20 °C |
| vapor pressure | 1 mm Hg ( 865 °C) |
| refractive index | n |
| Flash point: | 1413°C |
| storage temp. | +15C to +30C |
| solubility | H2O: soluble |
| form | tablets |
| appearance | Colorless crystals |
| color | White |
| Specific Gravity | 2.165 |
| PH | 5.5-6.5(1 tablet in 100 mL purified water) |
| Water Solubility | 360 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.01 |
| Merck | 14,8599 |
| BRN | 3534976 |
| Dielectric constant | 5.9(Ambient) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 7647-14-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium chloride(7647-14-5) |
| EPA Substance Registry System | Sodium chloride (7647-14-5) |
Safety information for Sodium chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P403:Store in a well-ventilated place. |
Computed Descriptors for Sodium chloride
| InChIKey | FAPWRFPIFSIZLT-UHFFFAOYSA-M |
Sodium chloride manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran




You may like
-
 Sodium Chloride 98%View Details
Sodium Chloride 98%View Details -
 SODIUM CHLORIDE IP 99%View Details
SODIUM CHLORIDE IP 99%View Details -
 Sodium Chloride 99%View Details
Sodium Chloride 99%View Details -
 Sodium chloride 99%View Details
Sodium chloride 99%View Details -
 Sodium chloride 98%View Details
Sodium chloride 98%View Details -
 Sodium chloride, 98% 99%View Details
Sodium chloride, 98% 99%View Details -
 Sodium chloride, 99% 99%View Details
Sodium chloride, 99% 99%View Details -
 Sodium chloride solution CASView Details
Sodium chloride solution CASView Details
