Magnesium chloride
Synonym(s):Magnesium chloride;Magnesium chloride hexahydrate;Magnesium dichloride;Magnogene;PCR optimization
- CAS NO.:7786-30-3
- Empirical Formula: Cl2Mg
- Molecular Weight: 95.21
- MDL number: MFCD00149782
- EINECS: 232-094-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:27:04
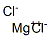
What is Magnesium chloride?
Absorption
Oral: Inversely proportional to amount ingested; 40% to 60% under controlled dietary conditions; 15% to 36% at higher doses
Toxicity
Mouse LD50 775mg/kg (intraperitoneal) Mouse LD50 : 7600mg/kg (oral) Rat LD 50 : 8100mg/kg (oral) Rat LD50 176mg/kg (intravenous) Severe toxicity occurs most often after intravenous infusions. It can also occur after chronic excessive oral doses, often in patients with renal insufficiency. Early manifestations are lethargy, hyporeflexia, followed by weakness, paralysis, hypotension, ECG changes (prolonged PR and QRS intervals), CNS depression, seizures, and respiratory depression. In overdose, magnesium impairs neuromuscular transmission, manifested as weakness and hyporeflexia.
Chemical properties
Magnesium is an essential nutrient for plants and animals and is a natural constituent of fruits, vegetables, grain, meats and sea-foods. It is the fourth most abundant cation in the human body and the second most plentiful intracellularly. The average 60-kg human adult body contains about 24 g magnesium of which about one-half resides in bone. Although plasma levels vary between 1.7 and 3.0 mg per dl, very little is contained in extracellular fluid. Magnesium is essential for the production and transfer of energy, for protein, fat, and nucleic acid synthesis, for contractility in muscle and excitability in nerve, and for the activity of numerous enzyme systems.
Occurrence
MgCl2, is one of the primary constituents of seawater and occurs in most natural brines and salt deposits formed from the evaporation of seawater.
The Uses of Magnesium chloride
Beyond magnesium metal production, magnesium chloride is widely used in textiles, paper, fireproofing agents, cements, and refrigeration brine, as well as for dust and erosion control. When mixed with magnesium oxide, it forms a hard material known as Sorel cement (approximate formula Mg?Cl?(OH)?(H?O)?), a unique application among compounds. It also served as an early antiseptic and has shown some antitumor efficacy as a feed additive in veterinary studies.
Commercial liquid MgCl? products (e.g., DustGard Liquid) are used for dust suppression on unpaved roads and construction sites, offering environmental benefits and reduced maintenance costs. Medically, it is a highly bioavailable magnesium supplement with minimal laxative effects and is used as an anesthetic for cephalopods and bivalves. In food processing, it is a key coagulant in tofu production, known as “Nigari” in Japan and “Lushui” in China, and is also an ingredient in infant formula. Additionally, MgCl? shows potential for hydrogen storage by adsorbing ammonia to form Mg(NH?)?Cl?, which releases hydrogen upon mild heating and catalysis.
Background
Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water.
Indications
Magnesium chloride is used in several medical and topical (skin related) applications. Magnesium chloride usp, anhydrous uses as electrolyte replenisher, pharmaceutic necessity for hemodialysis and peritoneal dialysis fluids.
What are the applications of Application
Magnesium chloride is an excellent magnesium ion source for synthesis and biology research
Definition
ChEBI: Magnesium chloride is a magnesium salt comprising of two chlorine atoms bound to a magnesium atom.
Preparation
In the "Dow Process"—an electrolytic method for extracting bromine from brine—magnesium chloride is regenerated from magnesium hydroxide using hydrochloric acid: Mg(OH)?(s) + 2HCl → MgCl?(aq) + 2H?O. It can also be prepared from magnesium carbonate via a similar reaction. As an ionic compound, molten magnesium chloride undergoes electrolysis with electric current carried by ion movement and electrode discharge, while solid magnesium chloride does not conduct electricity due to immobile ions.
General Description
Magnesium chloride (MgCl2) is an inorganic compound that can be prepared by reacting magnesium oxide and ammonium chloride in the presence of alumina as a covering agent.
Hazard
Toxic by ingestion.
Flammability and Explosibility
Non flammable
Pharmacokinetics
Magnesium is important as a cofactor in many enzymatic reactions in the body involving protein synthesis and carbohydrate metabolism (at least 300 enzymatic reactions require magnesium). Actions on lipoprotein lipase have been found to be important in reducing serum cholesterol and on sodium/potassium ATPase in promoting polarization (eg, neuromuscular functioning).
Metabolism
Magnesium levels are efficiently regulated by the kidneys. Magnesium also undergoes efficient enterohepatic circulation
Properties of Magnesium chloride
| Melting point: | 714 °C (lit.) |
| Boiling point: | 1412 °C/1 atm (lit.) |
| Density | 2.32 g/mL at 25 °C (lit.) |
| refractive index | n20/D 1.336 |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | powder |
| color | colorless |
| Specific Gravity | 2.41 |
| Odor | odorless |
| PH | 5.0-7.5 (25℃, 1M in H2O) |
| Water Solubility | 400 G/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.03 |
| Merck | 14,5662 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7786-30-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium dichloride(7786-30-3) |
| EPA Substance Registry System | Magnesium chloride (7786-30-3) |
Safety information for Magnesium chloride
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Magnesium chloride
Magnesium chloride manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 MAGNESIUM CHLORIDE 99%View Details
MAGNESIUM CHLORIDE 99%View Details -
 Magnesium Chloride 99%View Details
Magnesium Chloride 99%View Details -
 Magnesium Chloride 99%View Details
Magnesium Chloride 99%View Details -
 Magnesium chloride 98%View Details
Magnesium chloride 98%View Details -
 Magnesium chloride CAS 7786-30-3View Details
Magnesium chloride CAS 7786-30-3View Details
7786-30-3 -
 Magnesium chloride, ultra dry CAS 7786-30-3View Details
Magnesium chloride, ultra dry CAS 7786-30-3View Details
7786-30-3 -
 Magnesium chloride, Ultra dry CAS 7786-30-3View Details
Magnesium chloride, Ultra dry CAS 7786-30-3View Details
7786-30-3 -
 magnesium chloride CASView Details
magnesium chloride CASView Details
