Triethanolamine
Synonym(s):2,2′,2′′-Nitrilotriethanol;Triethanolamine;Tris(2-hydroxyethyl)amine;Tris(2-hydroxyethyl)amine, 2,2′,2′′-Trihydroxytriethylamine, TEA
- CAS NO.:102-71-6
- Empirical Formula: C6H15NO3
- Molecular Weight: 149.19
- MDL number: MFCD00002855
- EINECS: 203-049-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:27:12
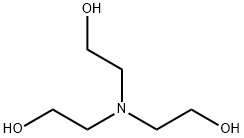
What is Triethanolamine?
Absorption
Dermal absorption of trolamine increases with the dose . This has been found to range from 19-28% in rats with doses of 68-276 mg/kg in 190 μL of acetone without occlusion and from 60-80% in mice with doses of 79-1120 mg/kg in the same volume of acetone.
Toxicity
Trolamine produced mild erythema and edema in rabbits with dermally administered doses of 2 g/kg under occlusion . The oral LD50 was found to be 8 g/kg in guinea pigs and 4.19-11.26 g/kg in rats. Repeated oral administration in rats and guinea pigs produced hepatic and renal damage with deaths occurring in rats at doses >0.3 g/kg/day. When inhaled trolamine produced edema and inflammation in rats at doses over 100 mg/m3 after 5 days and at doses over 4.7 mg/m3 after 90 days. There is some evidence of carcinogenicity at high dermal doses (1000 mg/kg/day) in mice. Trolamine is not classified as a carcinogen in humans.
Chemical properties
Triethanolamine is a pale yellow and viscous liquid. It is hygroscopic with an irritant and ammoniacal odor. Triethanolamine is incompatible with copper, copper alloys, galvanised iron, acids, and oxidisers. There are multiple industrial and domestic applications for this compound, i.e., in the manufacture of toilet products, cosmetics formulations, solvents for waxes, resins, dyes, paraffi ns and polishes, herbicides, and lubricants for textile products.
The Uses of Triethanolamine
In industries, triethanolamine is used as a corrosion inhibitor in metal-cutting fluids; a curing agent for epoxy and rubber polymers; a copper–triethanolamine; in emulsifiers, thickeners, and wetting agents in the formulation of consumer products such as cosmetics, detergents, shampoos, and other personal products; and a neutraliser-dispersing agent in agricultural herbicide formulations. In brief, triethanolamine has wide applications as a corrosion inhibitor, a surface-active agent, and an intermediate in various products including metalworking fluids, oils, fuels, paints, inks, cement, cosmetic, and personal products and formulations of algicides and herbicides.
Background
Trolamine, or triethanolamine (TEA), is a tertiary amine and a triol. It is a bifunctional compound that exhibits both properties of alcohols and amines. Trolamine contains small amounts of diethanolamine and ethanolamine and may also act as an antioxidant against the auto-oxidation of animal and vegetable fats. It is commonly used as a pH adjuster and surfactant in industrial and cosmetic products such as skin and hair conditioning.
Indications
Trolamine is used as an alkalizing agent, surfactant, and counter-ion in cosmetic and pharmaceutical formulations . It is not considered to be an active pharmacological ingredient and so has no official indication.
Definition
ChEBI: Triethanolamine is a tertiary amino compound that is ammonia in which each of the hydrogens is substituted by a 2-hydroxyethyl group. It has a role as a buffer and a surfactant. It is a tertiary amino compound, a triol and an amino alcohol. It is functionally related to a triethylamine. It is a conjugate base of a triethanolammonium.
Preparation
Triethanolamine is prepared commercially by the ammonolysis of ethylene oxide. The reaction yields a mixture of monoethanolamine, diethanolamine, and triethanolamine, which are separated to obtain the pure products.
World Health Organization (WHO)
Trolamine is widely used as an emulsifier in combination with fatty acids in pharmaceutical and cosmetic products. The World Health Organization is not aware of restrictive action having been taken elsewhere.
Reactivity Profile
Triethanolamine is an aminoalcohol. Neutralize acids to form salts plus water in exothermic reactions. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides. Reacts violently with strong oxidants. [Handling Chemicals Safely 1980. p. 928].
Health Hazard
Exposures to triethanolamine, in contrast with other chemical compounds, is known to cause low toxicity to animals and the acute oral LD50 to rats and guinea pigs ranges from 8000 to 9000 mg/kg. Triethanolamine was found to be a moderate eye irritant. A 5%–10% solution of triethanolamine did not induce skin irritation or skin sensitization. Studies of Inoue et al. and many other workers have indicated the absence of the mutagenic potential of triethanolamine as evidenced by both in vivo and in vitro studies (Salmonella typhimurium tests, Chinese hamster ovary cells, and rat liver chromosome analysis). Further, extensive studies have demonstrated the absence of potential carcinogenicity of triethanolamine in rats and mice, suggesting a low or lack of acute or chronic toxicity of the chemical to mammals.
Fire Hazard
Special Hazards of Combustion Products: Poisonous gases, such as NOx, may be produced
Contact allergens
This emulsifying agent can be contained in many products such as cosmetics, topical medicines, metalworking cut- ting fluids, and color film developers. Traces may exist in other ethanolamines such as monoand diethanolamine. Contact allergy seems to be rarer than previously thought.
Pharmacokinetics
Acts as a surfactant or alkalizing agent to aid in emulsification and solubilizing of compounds or in raising the pH of a solution
Toxicity
Triethanolamine is used primarily as an emulsifying agent in a
variety of topical pharmaceutical preparations. Although generally
regarded as a nontoxic material, triethanolamine may cause
hypersensitivity or be irritant to the skin when present in formulated
products. The lethal human oral dose of triethanolamine is
estimated to be 5–15 g/kg body-weight.
Following concern about the possible production of nitrosamines
in the stomach, the Swiss authorities have restricted the use of
triethanolamine to preparations intended for external use.
LD50 (guinea pig, oral): 5.3 g/kg
LD50 (mouse, IP): 1.45 g/kg
LD50 (mouse, oral): 7.4 g/kg
LD50 (rat, oral): 8 g/kg
Potential Exposure
Monoethanolamine is widely used in industry for scrubbing acid gases and in production of detergents and alkanolamide surfactants; to remove carbon dioxide and hydrogen from natural gas, to remove hydrogen sulfide and carbonyl sulfide; as an alkaline conditioning agent; as an intermediate for soaps, detergents, dyes, and textile agents. Diethanolamine is an absorbent for gases; a solubilizer for 2,4- dichlorophenoxyacetic acid (2,4-D); and a softener and emulsifier intermediate for detergents. It also finds use in the dye and textile industry. Triethanolamine is used as plasticizers, neutralizer for alkaline dispersions; lubricant additive; corrosion inhibitor; and in the manufacture of soaps, detergents, shampoos, shaving preparations; face and hand creams; cements, cutting oils, insecticides, surface active agents; waxes, polishes, and herbicides.
Carcinogenicity
Results of carcinogenicity studies have been controversial. Hoshino and Tanooka reported that triethanolamine in the diet of mice at levels of 0.03% or 0.3% caused a significant increase in the occurrence of tumors, both benign and malignant. Females showed a 32% increase, mostly of thymic lymphomas. The increase of all other tumors, in both sexes, was 8.2%. They also found that triethanolamine reacted with sodium nitrite to produce N-nitrosodiethanolamine and that the product caused mutagenesis in bacteria. Maekawa et al. reported that no carcinogenic activity was found when given orally to rats in drinking water at concentrations of 1% and 2% for 2 years. However, the dosage to females was halved after week 69 of treatment owing to nephrotoxicity. Histological examination of renal damage in treated animals revealed acceleration of chronic nephropathy, mineralization of the renal papilla, nodular hyperplasia of the pelvic mucosa, and pyelonephritis with or without papillary necrosis. Nephrotoxicity seemed to affect life span adversely, especially in females. Tumor incidence and histology were the same in the treated group as in controls.
Metabolism
Trolamine is excreted mostly as the unchanged compound . No diethanolamine or ethanolamine has been found. Very small amounts of trolamine glucuronide have been detected but not quantified.
Storage
Triethanolamine may turn brown on exposure to air and light.
The 85% grade of triethanolamine tends to stratify below 15℃;
homegeneity can be restored by warming and mixing before use.
Triethanolamine should be stored in an airtight container
protected from light, in a cool, dry place.
See Monoethanolamine for further information.
Incompatibilities
Triethanolamine is a tertiary amine that contains hydroxy groups; it
is capable of undergoing reactions typical of tertiary amines and alcohols. Triethanolamine will react with mineral acids to form
crystalline salts and esters. With the higher fatty acids, triethanolamine
forms salts that are soluble in water and have characteristics
of soaps. Triethanolamine will also react with copper to form
complex salts. Discoloration and precipitation can take place in the
presence of heavy metal salts.
Triethanolamine can react with reagents such as thionyl chloride
to replace the hydroxy groups with halogens. The products of these
reactions are very toxic, resembling other nitrogen mustards.
Waste Disposal
Controlled incineration; incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions
Regulatory Status
Included in the FDA Inactive Ingredients Database (rectal, topical, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Triethanolamine
| Melting point: | 17.9-21 °C (lit.) |
| Boiling point: | 190-193 °C/5 mmHg (lit.) |
| Density | 1.124 g/mL at 25 °C (lit.) |
| vapor density | 5.14 (vs air) |
| vapor pressure | 0.01 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 365 °F |
| storage temp. | Store at RT. |
| solubility | H2O: 1 M, clear, colorless |
| form | Oily Liquid |
| color | Clear colorless to slightly yellow |
| Specific Gravity | 1.125 (20/20℃) |
| PH | 10.5-11.5 (25℃, 1M in H2O) |
| pka | 7.8(at 25℃) |
| PH Range | 7.3 - 8.3 |
| Odor | Mild ammoniacal. |
| explosive limit | 3.6-7.2%(V) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive & Hygroscopic |
| λmax | λ: 280 nm Amax: 0.1 |
| Merck | 14,9665 |
| BRN | 1699263 |
| Exposure limits | ACGIH: TWA 5 mg/m3 |
| Dielectric constant | 6.9(40℃) |
| CAS DataBase Reference | 102-71-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Triethanolamine(102-71-6) |
| IARC | 3 (Vol. 77) 2000 |
| EPA Substance Registry System | Triethanolamine (102-71-6) |
Safety information for Triethanolamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triethanolamine
| InChIKey | GSEJCLTVZPLZKY-UHFFFAOYSA-N |
Triethanolamine manufacturer
JKM Chemtrade
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 Triethanol amine 98%View Details
Triethanol amine 98%View Details -
 Triethanol Amine 99%View Details
Triethanol Amine 99%View Details -
 TRI ETHANOL AMINE 99%View Details
TRI ETHANOL AMINE 99%View Details -
 Triethanolamine CASView Details
Triethanolamine CASView Details -
 Triethanolamine (SQ) CAS 102-71-6View Details
Triethanolamine (SQ) CAS 102-71-6View Details
102-71-6 -
 Triethanolamine CASView Details
Triethanolamine CASView Details -
 Liquid Tri ethanolamine ChemicalView Details
Liquid Tri ethanolamine ChemicalView Details
102-71-6 -
 5 Kg Liquid Triethanolamine Tea, For Laboratory, 99%View Details
5 Kg Liquid Triethanolamine Tea, For Laboratory, 99%View Details
102-71-6
