Sodium carbonate
Synonym(s):Sodium carbonate;Soda ash;Soda;Sodium carbonate solution;anhydrous soda
- CAS NO.:497-19-8
- Empirical Formula: CH2O3.2Na
- Molecular Weight: 105.99
- MDL number: MFCD00003499
- EINECS: 207-838-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-26 13:56:28
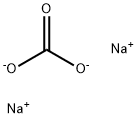
What is Sodium carbonate?
Absorption
The uptake of sodium, via exposure to sodium carbonate, is much less than the uptake of sodium via food. Therefore, sodium carbonate is not expected to be systemically available in the body. Furthermore, an oral uptake of sodium carbonate will result in a neutralization in the stomach due to the gastric acid.
Toxicity
Man: LD50 (Oral) - 714 mg/kg, Effect: Behavioural,General Anesthetic : GI Ulceration or Bleeding from small intestine. Mouse : LC50 ( Inhalation ) - 1200mg/m3/2h : GI Other Change Mouse : LC50 ( Intraperitoneal ) - 117mg/kg Mouse : LD50 ( Oral) - 6600mg/kg Mouse : LD50 (Subcutaneous ) - 2210 mg/kg Rat : LC50 ( Inhalation ) 2300mg/m3/2H Rat: LD50 (Oral) - 4090 mg/kg
Description
Sodium carbonate, also known as soda ash or washing soda, is a widely used inorganic compound. It is primarily obtained naturally from trona, a sodium carbonate-bicarbonate mineral. Major deposits of trona are found in Wyoming, Egypt’s Nile Valley, and California. Soda ash is produced by crushing and heating trona ore, then purifying the resulting mixture through dissolving and filtering processes.
Description
Sodium carbonate (Na2CO3) is a moderately strong base used commonly in organic chemistry. It is regularly employed as the base of choice for palladium catalyzed reactions such as Suzuki couplings. Sodium carbonate is also used for other reactions that require a base to neutralize acids that form during reactions. For example, alkylation reactions often employ sodium carbonate to mop up protons (acid) that forms during the reaction.
The Uses of Sodium carbonate
Sodium carbonate is an alkali that appears as crystals or crystalline powder and dissolves easily in water. It serves multiple functions, including as an antioxidant, curing and pickling agent, flavoring agent, processing aid, sequestrant, and pH control agent. It is used in instant soups to neutralize acidity and in alginate water dessert gels to sequester calcium, allowing the alginate to dissolve. It is also used in puddings, sauces, and baked goods.
What are the applications of Application
Sodium carbonate, anhydrous is used in chromatography and protein isolation techniques
Indications
Used topically for dermatitides, mouthwash, vaginal douche; veterinary use as emergency emetic.Occasionally, for dermatitides topically as a lotion. Medication (Vet): In solution to cleanse skin, in eczema, to soften scabs of ringworm.
Background
Sodium Carbonate is the disodium salt of carbonic acid with alkalinizing property. When dissolved in water, sodium carbonate forms carbonic acid and sodium hydroxide. As a strong base, sodium hydroxide neutralizes gastric acid thereby acting as an antacid.
Production Methods
Sodium carbonate is produced globally from its minerals, with significant deposits found in Africa and the United States as carbonate or trona, a mixed ore of carbonate and bicarbonate. About 70% of global production is made via the Solvay process, where ammonia is added to a sodium chloride solution, and carbon dioxide is bubbled through to precipitate bicarbonate, which is then decomposed by heat to produce sodium carbonate. In the United States, production is solely based on minerals containing sodium carbonate. Different grades of sodium carbonate are produced, including technical, food, and pharmaceutical grades.
Properties of Sodium carbonate
| Melting point: | 851 °C (lit.) |
| Boiling point: | 1600°C |
| Density | 2.53 |
| refractive index | 1.535 |
| storage temp. | 15-25°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| appearance | White powder |
| color | White |
| Specific Gravity | 2.532 |
| Odor | at 100.00?%. odorless |
| PH | 10.52(1 mM solution);10.97(10 mM solution);11.26(100 mM solution); |
| pka | (1) 6.37, (2) 10.25 (carbonic (at 25℃) |
| Water Solubility | 22 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,8596 |
| BRN | 4154566 |
| Dielectric constant | 5.3(Ambient) |
| Stability: | Stable. Incompatible with powdered alkaline earth metals, aluminium, organic nitro compounds, fluorine, alkali metals, nonmetallic oxides, concentrated sulfuric acid, oxides of phosphorus. |
| CAS DataBase Reference | 497-19-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium carbonate(497-19-8) |
| EPA Substance Registry System | Sodium carbonate (497-19-8) |
Safety information for Sodium carbonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Sodium carbonate
| InChIKey | CDBYLPFSWZWCQE-UHFFFAOYSA-L |
Sodium carbonate manufacturer
Krishna Chemicals
New Products
N,O-Dimethylhydroxylamine hydrochloride DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide 3-Hydroxypropionitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran





You may like
-
 Sodium carbonate anhydrous 99%View Details
Sodium carbonate anhydrous 99%View Details -
 Sodium carbonate 99%View Details
Sodium carbonate 99%View Details -
 Sodium carbonate 98%View Details
Sodium carbonate 98%View Details -
 Sodium carbonate, anhydrous CAS 497-19-8View Details
Sodium carbonate, anhydrous CAS 497-19-8View Details
497-19-8 -
 SODIUM CARBONATE SOLUTION 1.0 N 99%View Details
SODIUM CARBONATE SOLUTION 1.0 N 99%View Details -
 Sodium Carbonate CASView Details
Sodium Carbonate CASView Details -
 Benedict`s Reagent Quantitative CASView Details
Benedict`s Reagent Quantitative CASView Details -
 BENEDICT'S REAGENT (QUANTITATIVE) CASView Details
BENEDICT'S REAGENT (QUANTITATIVE) CASView Details
