Methanol
Synonym(s):Methanol;Methyl alcohol;Methanol in DMSO;Methanol solution;Methanol ZerO2
- CAS NO.:67-56-1
- Empirical Formula: CH4O
- Molecular Weight: 32.04
- MDL number: MFCD00004595
- EINECS: 200-659-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:05

What is Methanol?
Description
Methanol, also known as methyl alcohol or wood alcohol, is the simplest alcohol, appearing as a clear, colorless, and flammable liquid. It is fully miscible with water, ethanol, and ether, burns with a pale blue flame producing water and carbon dioxide, and forms explosive vapor-air mixtures at concentrations of 6.0–36.5% by volume. Highly toxic to the nervous system—particularly the retina—it is used in chemical production, removal of water from automotive and aviation fuels, as a solvent for paints and plastics, and as an ingredient in a wide range of products.
Chemical properties
Methanol is a clear, water-white liquid with a mild odor at ambient temperatures. Its odor threshold in air has been reported as approximately 100 ppm, though some studies indicate concentrations of 2000 to 5900 ppm are barely detectable.
The Uses of Methanol
Methanol is an inexpensive and important synthetic raw material widely used in the organic chemical industry. Nearly half of its production is dedicated to formaldehyde manufacturing. Other applications include use as an automotive antifreeze and fuel, an intermediate in synthetic protein production, and a precursor for acetic acid, methyl tert-butyl ether, and various chemical intermediates. It also serves as an octane improver (in oxinol), a potential alternative to diesel fuel, and—due to its excellent polar solvent properties—a common laboratory chemical and methylating reagent.
Definition
ChEBI: Methanol is the primary alcohol that is the simplest aliphatic alcohol, comprising a methyl and an alcohol group. It has a role as an amphiprotic solvent, a fuel, a human metabolite, an Escherichia coli metabolite, a mouse metabolite and a Mycoplasma genitalium metabolite. It is an alkyl alcohol, a one-carbon compound, a volatile organic compound and a primary alcohol. It is a conjugate acid of a methoxide.
Production Methods
Modern industrial-scale methanol production is exclusively based on synthesis from pressurized mixtures of hydrogen, carbon monoxide, and carbon dioxide gases in the presence of catalysts. Based on production volume, methanol has become one of the largest commodity chemicals produced in the world.
World Health Organization (WHO)
Methanol has been subjected to abuse by consumption as a substitute for ethanol. Its toxic metabolites cause irreversible blindness and severe metabolic acidosis, and are ultimately fatal. Methanol continues to be used as an industrial solvent.
Reactivity Profile
Methanol reacts violently or explosively with various substances: it reacts intensely with acetyl bromide; mixtures with concentrated sulfuric acid and hydrogen peroxide can cause explosions; reactions with hypochlorous acid (in aqueous or carbon tetrachloride solution) or chlorine yield methyl hypochlorite, which decomposes and may explode upon exposure to light or heat; it reacts explosively with isocyanates under basic conditions (mitigated by inert solvents); exhibits violent exothermic reaction with bromine; solutions of anhydrous lead perchlorate in methanol explode when disturbed; reacts violently with P?O?; and can ignite on contact with a platinum-black catalyst.
Hazard
Flammable, dangerous fire risk. Explosive limits in air 6–36.5% by volume. Toxic by ingestion (causes blindness). Headache, eye damage, dizziness, and nausea.
Potential Exposure
Drug,Mutagen; Reproductive Effector; Human Data; PrimaryIrritant. Methyl alcohol is used as a starting material inorganic synthesis of chemicals, such as formaldehyde,methacrylates, methyl amines, methyl halides, ethylene glycol, and pesticides, and as an industrial solvent for inks, resins, adhesives, and dyes. It is an ingredient in paint and varnish removers, cleaning and dewaxing preparations, spirit duplicating fluids, embalming fluids, antifreeze mixtures, and enamels, and is used in the manufacture of photographic film, plastics, celluloid, textile soaps, wood stains, coated fabrics, shatterproof glass, paper coating, waterproofing formulations, artificial leather, and synthetic indigo and other dyes. It has also been used as an extractant in many other processes, an antidetonant fuel-injection fluid for aircraft, a rubber accelerator, and a denaturant for ethyl alcohol.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Source
Methanol occurs naturally in various plants including small-flowered oregano (5-45 ppm), Guveyoto shoots (700 ppb), orange juice (0.8-80 ppm), onion bulbs, pineapples, black currants, spearmint, apples, jimsonweed leaves, soybean plants, wild parsnip, blackwood, soursop, cauliflower, caraway, petitgrain, bay leaves, tomatoes, parsley leaves, and geraniums. It may also enter the environment through spills from formaldehyde solutions where it is used to prevent polymerization.
Toxicity evaluation
The toxic properties of methanol are the result of accumulation
of the formate intermediate in the blood and tissues of exposed
individuals. Formate accumulation produces metabolic
acidosis leading to the characteristic ocular toxicity (blindness)
observed in human methanol poisonings.
Humans and primates appear particularly sensitive to
methanol toxicity when compared to rats. This is attributed to
the slower rate of conversion in humans of the formate
metabolite via tetrahydrofolate. This step in methanol metabolism
occurs in rats at a rate ~2.5 times that observed in
humans.
Formate appears to directly affect the retina and optic nerve
by acting as a mitochondrial toxin. It is believed that formate
acts as a metabolic poison by inhibiting cytochrome oxidase
activity. The cells of the optic nerve have low reserves of cytochrome
oxidase and thus may be particularly sensitive to
formate-induced metabolic inhibition.
Incompatibilities
Methanol reacts violently with strong oxidizers, causing a fire and explosion hazard.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration
Properties of Methanol
| Melting point: | -98 °C(lit.) |
| Boiling point: | 65.4 °C(lit.) |
| Density | 0.791 g/mL at 25 °C |
| vapor density | 1.11 (vs air) |
| vapor pressure | 410 mm Hg ( 50 °C) |
| refractive index | n |
| Flash point: | 52 °F |
| storage temp. | 2-8°C |
| solubility | benzene: miscible(lit.) |
| form | Liquid Free From Particulates |
| appearance | Colorless liquid |
| pka | 15.2(at 25℃) |
| Specific Gravity | 0.793 (20/20℃) |
| color | <10(APHA) |
| Odor | Faint alcohol odor detectable at 4 to 6000 ppm (mean = 160 ppm) |
| Relative polarity | 0.762 |
| explosive limit | 5.5-44%(V) |
| Odor Threshold | 33ppm |
| Water Solubility | miscible |
| λmax | λ: 210 nm Amax: 0.50 λ: 220 nm Amax: 0.30 λ: 230 nm Amax: 0.15 λ: 235 nm Amax: 0.10 λ: 240 nm Amax: 0.05 λ: 260 nm Amax: 0.01 λ: 400 nm Amax: 0.01 |
| Merck | 14,5957 |
| BRN | 1098229 |
| Henry's Law Constant | 4.99 at 25 °C (headspace-GC, Gupta et al., 2000) |
| Exposure limits | TLV-TWA (200 ppm) (ACGIH), 260mg/m3, 1040mg/m3 (800 ppm) 15minutes (NIOSH); STEL 310mg/m3 (250 ppm); IDLH 25,000 ppm (NIOSH). |
| Dielectric constant | 33.6(20℃) |
| CAS DataBase Reference | 67-56-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Methyl alcohol(67-56-1) |
| EPA Substance Registry System | Methanol (67-56-1) |
Safety information for Methanol
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Methanol
Methanol manufacturer
New Products
tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-Arg-OH HCl H2O Indole Methyl Resin 2-CTC Resin Gabapentin EP Impurity A 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 1,3-Diphenylurea 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID N-METHYL INDAZOLE-3-CARBOXYLIC ACID 2-Hydroxy-5-nitroacetophenone 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2-HYDROXY BENZYL CYANIDE DIETHYL AMINOMALONATE HYDROCHLORIDE 5-BROMO-2CYANO PYRIDINE Boldenone Clarithromycin Ethinyl Estradiol StanozololRelated products of tetrahydrofuran
You may like
-
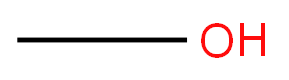 Methanol 98%View Details
Methanol 98%View Details -
 Methanol 99%View Details
Methanol 99%View Details -
 Methanol 99%View Details
Methanol 99%View Details -
 Methanol 98%View Details
Methanol 98%View Details -
 Methanol 98%View Details
Methanol 98%View Details -
 Methanol CAS 67-56-1View Details
Methanol CAS 67-56-1View Details
67-56-1 -
 Methanol CAS 67-56-1View Details
Methanol CAS 67-56-1View Details
67-56-1 -
 Methanol CAS 67-56-1View Details
Methanol CAS 67-56-1View Details
67-56-1
