Benzyl alcohol
Synonym(s):Benzenemethanol;BnOH;Phenylcarbinol
- CAS NO.:100-51-6
- Empirical Formula: C7H8O
- Molecular Weight: 108.14
- MDL number: MFCD00004599
- EINECS: 202-859-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-15 13:03:24
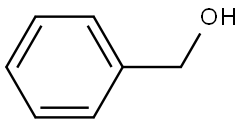
What is Benzyl alcohol?
Toxicity
1250 mg/kg (rat, oral) LD50 400 mg/kg IPR-RAT LD50 2000 mg/kg SKN-RBT LD50 53 mg/kg IVN-RAT LD50 2500 mg/kg ORL-GPG LD50
Description
Benzyl alcohol serves as a co-catalyst for epoxy resins and is also a component of the color developer C-22.
Chemical properties
Benzyl alcohol, a colorless liquid with a faint, sweet aroma, occurs naturally in many essential oils and foods. It can be oxidized to benzaldehyde, such as with nitric acid, or dehydrogenated over a copper-magnesium oxide-pumice catalyst. Esterification of benzyl alcohol yields various important flavor and fragrance compounds. Diphenylmethane is produced through a Friedel-Crafts reaction between benzyl alcohol and benzene. Additionally, heating benzyl alcohol with strong acids or bases leads to the formation of dibenzyl ether.
The Uses of Benzyl alcohol
A solution of alcohol (A) (86 mg, 482 μmol) in anhydrous THF (5 mL) was treated with NaH (60% dispersion in mineral oil, 21 mg, 526 μmol) at room temperature and stirred for 15 minutes. Aryl chloride (B) (150 mg, 439 μmol) was then added, and the resulting mixture was stirred at room temperature for 45 minutes, followed by heating at 80 °C for 2 hours. After completion, the reaction was quenched with water and saturated aqueous NaHCO?, extracted with EtOAc, washed with brine, dried (Na?SO?), and concentrated. The crude material was purified by silica gel column chromatography (0–25% EtOAc/cyclohexane) to afford the product.
Pharmaceutical Applications
Benzyl alcohol is widely used as an antimicrobial preservative in cosmetics, foods, and various pharmaceutical formulations—including oral and parenteral products—at concentrations typically around 1% v/v and up to 2.0% v/v. Although it has been employed in protein, peptide, and small molecule drugs, its usage frequency has declined over the years. In cosmetics, concentrations up to 3.0% v/v may be used for preservation, while higher concentrations serve as solubilizers (≥5% v/v) or disinfectants (10% v/v). The 10% v/v solution also exhibits local anesthetic properties, making it useful in certain parenteral, cough, ophthalmic, and dermatological products. However, due to associations with fatal adverse reactions in neonates, it is recommended that benzyl alcohol-preserved parenteral products be avoided in newborns whenever possible.
Background
Benzyl alcohol is a monoaromatic primary alcohol widely utilized as a solvent and intermediate in the cosmetics, pharmaceuticals, and flavor/fragrance industries. It can form an intramolecular OH-π hydrogen bond and predominantly adopts a gauche cis conformation around the alcoholic group.
Applications
Benzyl alcohol is an aromatic alcohol used in a wide range of cosmetic formulations as a fragrance component, preservative, solvent, and viscosity-decreasing agent. It can react with triethyl phosphite and zinc iodide to form the corresponding phosphonates, and also serves as a precursor in the synthesis of benzyl bromoacetate via reaction with bromoacetyl bromide in the presence of sodium hydrogen carbonate.
Safety
Benzyl alcohol was deemed safe for use in hair dyes at concentrations up to 10%. This conclusion took into account its limited body exposure, duration of use, and frequency of application, indicating that non-immunological reactions are not a concern. However, due to the wide range of product types containing this ingredient, inhalation may also serve as a potential route of exposure.
Storage
Benzyl alcohol slowly oxidizes in air to form benzaldehyde and benzoic acid, but does not react with water. Aqueous solutions can be sterilized by filtration or autoclaving, though some may produce benzaldehyde during autoclaving. It should be stored in metal or glass containers—avoiding plastic unless made of polypropylene or coated with inert fluorinated polymers like Teflon—under airtight conditions, protected from light, in a cool and dry place.
Purification Methods
Benzyl alcohol is typically purified by careful fractional distillation under reduced pressure in the absence of air. Benzaldehyde impurities can be detected by UV absorption at 283 nm. Common purification methods include shaking with aqueous KOH, extraction with peroxide-free diethyl ether, followed by washing with water, treatment with NaHS solution, filtration, drying over CaO, and finally distillation under reduced pressure. Alternatively, peroxy compounds can be removed by shaking with an Fe2? solution, washing the alcohol layer with water, and fractional distillation.
Properties of Benzyl alcohol
| Melting point: | -15 °C |
| Boiling point: | 205 °C |
| Density | 1.045 g/mL at 25 °C(lit.) |
| vapor density | 3.7 (vs air) |
| vapor pressure | 13.3 mm Hg ( 100 °C) |
| FEMA | 2137 | BENZYL ALCOHOL |
| refractive index | n |
| Flash point: | 201 °F |
| storage temp. | Store at +2°C to +25°C. |
| solubility | H2O: 33 mg/mL, clear, colorless |
| form | Liquid |
| appearance | Colorless liquid |
| pka | 14.36±0.10(Predicted) |
| color | APHA: ≤20 |
| Odor | Mild, pleasant. |
| Relative polarity | 0.608 |
| explosive limit | 1.3-13%(V) |
| Water Solubility | 4.29 g/100 mL (20 ºC) |
| Merck | 14,1124 |
| JECFA Number | 25 |
| BRN | 878307 |
| Henry's Law Constant | <2.70 x 10-7 at 25 °C (thermodynamic method-GC/UV, Altschuh et al., 1999) |
| Exposure limits | No exposure limit is set. Because of its
low vapor pressure and low toxicity, the
health hazard to humans from occupational
exposure should be very low. |
| Dielectric constant | 13.1(20℃) |
| CAS DataBase Reference | 100-51-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzyl alcohol(100-51-6) |
| EPA Substance Registry System | Benzyl alcohol (100-51-6) |
Safety information for Benzyl alcohol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Benzyl alcohol
| InChIKey | WVDDGKGOMKODPV-UHFFFAOYSA-N |
Benzyl alcohol manufacturer
Akhil Healthcare Private Limited
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 BENZYL ALCOHOL 99%View Details
BENZYL ALCOHOL 99%View Details -
 BENZYL ALCOHOL 99%View Details
BENZYL ALCOHOL 99%View Details -
 Benzyl Alcohol 99%View Details
Benzyl Alcohol 99%View Details -
 BENZYL ALCOHOL 99%View Details
BENZYL ALCOHOL 99%View Details -
 Benzyl Alcohol 99%View Details
Benzyl Alcohol 99%View Details -
 Benzyl alcohol 98%View Details
Benzyl alcohol 98%View Details -
 Benzyl Alcohol CASView Details
Benzyl Alcohol CASView Details -
 Benzyl alcohol CASView Details
Benzyl alcohol CASView Details
