Acetonitrile
Synonym(s):ACN;Acetonitrile;Methyl cyanide;Acetonitrile solution;Cyanomethane
- CAS NO.:75-05-8
- Empirical Formula: C2H3N
- Molecular Weight: 41.05
- MDL number: MFCD00001878
- EINECS: 200-835-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:14:06
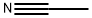
What is Acetonitrile ?
Description
Acetonitrile is a volatile liquid with an ether-like odor. As a highly polar solvent, it is widely used in industrial applications such as pharmaceuticals, photography, chemical manufacturing, and analytical industries. It serves as an effective solvent for separating olefins, polymers, spinning fibers, and plastics, and is also employed in copper extraction and refining, by-product ammonium sulfate recovery, textile dyeing, coating formulations, stabilizer for chlorinated solvents, perfume and cosmetics production, and as a general reagent in diverse chemical processes.
Chemical properties
Acetonitrile (CH?CN) is a colorless liquid with a sweet, ethereal odor. It is fully miscible with water, and its high dielectric constant and dipole moment make it an excellent solvent for inorganic, organic, and polymeric compounds. While widely used in the production of cosmetics, pharmaceuticals, and agricultural chemicals, it has been banned in cosmetic products in the European Economic Area since early 2000, with acetone and ethanol preferred as safer alternatives for household applications.
Physical properties
This colorless liquid has an ether-like or vinegar-like pungent odor. Dravnieks (1974) experimentally determined its detection odor threshold concentration to be 1,950 mg/m3 (1,161 ppmv), while Nagata and Takeuchi (1990) reported an odor threshold concentration of 13 ppmv.
The Uses of Acetonitrile
Acetonitrile, the simplest organic nitrile, is a by-product of acrylonitrile production and has largely replaced acrylonitrile in many applications. It serves primarily as an extraction solvent for butadiene, a chemical intermediate in pesticide synthesis, and a versatile solvent for both inorganic and organic compounds. It is also used to remove tars, phenols, and colorants from petroleum hydrocarbons, and finds applications in acrylic fiber manufacturing, pharmaceuticals, perfumes, nitrile rubber, ABS resins, HPLC and GC analysis, as well as copper extraction and refining. Furthermore, it acts as a starting material for producing acetophenone, α-naphthaleneacetic acid, thiamine, and acetamidine.
What are the applications of Application
Acetonitrile is used as a solvent for polymers, spinning fibers, plastic molding, and HPLC analysis; for the extraction of butadiene and other olefins from hydrocarbon streams; in textile dyeing and coating; and as a stabilizer for chlorinated solvents. It occurs naturally in coal tar and is formed as a by-product during acrylonitrile production. Although one of the more stable nitriles, it undergoes typical nitrile reactions and is employed in the synthesis of various nitrogen-containing compounds. It also serves as a catalyst and as a component in transition-metal complex catalysts.
Storage
Acetonitrile should only be used in ignition-free areas, and quantities exceeding 1 liter must be stored in tightly sealed metal containers separated from oxidizers.
Purification Methods
Commercial acetonitrile, a by-product of acrylonitrile production, contains impurities such as acrylonitrile, benzene, acetone, and water. A standard purification process involves: azeotropic distillation with methanol until clear distillate is obtained, reflux treatment with sodium hydride, filtration through acidic alumina, and dehydration/deacidification with calcium hydride followed by fractional distillation. Gas chromatography is recommended for impurity detection. Dehydration should avoid CaSO?/CaCl? and alkaline solutions; instead, use molecular sieves for preliminary drying followed by calcium hydride treatment. Trace P?O? may be added during distillation (while preventing polymerization) with final purification via K?CO? distillation to obtain high-purity product.
Properties of Acetonitrile
| Melting point: | -45 °C (lit.) |
| Boiling point: | 81-82 °C (lit.) |
| Density | 0.786 g/mL at 25 °C (lit.) |
| vapor density | 1.41 (vs air) |
| vapor pressure | 72.8 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 48 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | organic solvents: soluble(lit.) |
| form | liquid |
| appearance | Colorless liquid |
| pka | 25(at 25℃) |
| Specific Gravity | approximate 0.78(20/20℃) |
| color | <10(APHA) |
| Odor | Aromatic ether-like odor detectable at 40 ppm |
| Relative polarity | 0.46 |
| explosive limit | 3.0-17%(V) |
| Odor Threshold | 13ppm |
| Water Solubility | miscible |
| λmax | λ: 195 nm Amax: ≤0.12 λ: 200 nm Amax: ≤0.032 λ: 230 nm Amax: ≤0.0044 λ: 235 nm Amax: ≤0.0044 λ: 250 nm Amax: ≤0.0044 λ: 400 nm Amax: ≤0.0044 |
| Merck | 14,70 |
| BRN | 741857 |
| Henry's Law Constant | 7.30 at 5 °C, 8.90 at 10 °C, 11.6 at 15 °C, 14.6 at 20 °C, 17.6 at 25 °C (headspace-GC, Ji and
Evans, 2007) |
| Dielectric constant | 37.5(21℃) |
| Exposure limits | TLV-TWA 70 mg/m3 (40 ppm) (ACGIH and OSHA); STEL 105 mg/m3 (60 ppm) (ACGIH); IDLH 4000 ppm (NIOSH). |
| Stability: | Incompatible with alkali metals, acids, bases, reducing agents and oxidizing agents. Highly flammable. |
| CAS DataBase Reference | 75-05-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetonitrile(75-05-8) |
| EPA Substance Registry System | Acetonitrile (75-05-8) |
Safety information for Acetonitrile
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acetonitrile
Acetonitrile manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran




You may like
-
 Acetonitrile 99%View Details
Acetonitrile 99%View Details -
 Acetonitrile 99%View Details
Acetonitrile 99%View Details -
 27522 ACETONITRILE 99%View Details
27522 ACETONITRILE 99%View Details
27522 -
 Acetonitrile, GC Grade 99%View Details
Acetonitrile, GC Grade 99%View Details -
 Acetonitrile, 99% 99%View Details
Acetonitrile, 99% 99%View Details -
 Acetonitrile, HPLC Grade 99%View Details
Acetonitrile, HPLC Grade 99%View Details -
 Acetonitrile CASView Details
Acetonitrile CASView Details -
 Acetonitrile CASView Details
Acetonitrile CASView Details
