Thiophene-2-ethylamine
- CAS NO.:30433-91-1
- Empirical Formula: C6H9NS
- Molecular Weight: 127.21
- MDL number: MFCD06090846
- EINECS: 250-196-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:20:46
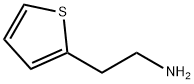
What is Thiophene-2-ethylamine?
Chemical properties
A colorless to yellow liquid with unstable properties requiring protection with nitrogen gas. It appears as a light yellow clear liquid, but turns red upon prolonged exposure.
The Uses of Thiophene-2-ethylamine
[2-(Thiophene-2-yl)ethyl]amine is used in the synthesis of geldanamycin derivatives as HCV replication inhibitors targetting Hsp90.
The Uses of Thiophene-2-ethylamine
2-Thiopheneethylamine (2-thiophene ethyl amine, 2-(thien-2-yl)ethylamine) is suitable to functionalize multiwall carbon nanotubes (MWCNT).
It may be used as a reactant in the synthesis of pyrimidine derivatives by reacting with various isothiocyanatoketones and acylguanidines derivatives by reacting with aroyl S-methylisothiourea.
General Description
2-Thiopheneethylamine (2-(thiophen-2-yl)ethanamine) is an aromatic amine. It undergoes microwave induced condensation with iminodiacetic acid to form the corresponding piperazine-2,6-dione derivatives. Its effect as a probable substitute to the pyridine ligand on the performance of poly(3-hexylthiophene)/CdSe hybrid solar cells has been investigated.
Synthesis
N,N-Dimethylformamide (DMF) reacts with thiophene to obtain 2-thiophenecarbaldehyde, then reacts with isopropyl chloroacetate to obtain 2-thiopheneacetaldehyde, and then reacts with hydroxylamine hydrochloride to obtain 2-thiopheneacetaldehyde oxime, and finally reduced to give 2-thiopheneethylamine.
References
[1] M. BARWIOLEK. Structural and spectral studies of silver(I) complexes with new Schiff bases derived from 2-thiopheneethylamine and their application in thin layer deposition by spin and dip coating techniques[J]. Polyhedron, 2017, 124: Pages 12-21. DOI:10.1016/j.poly.2016.12.011.
[2] BHUSHAN D. VARPE S B J. Schiff Base of Isatin with 2-Thiopheneethylamine and Its Mannich Bases: Synthesis, Docking, and In Vitro Anti-Inflammatory and Antitubercular Activity[J]. Russian Journal of Bioorganic Chemistry, 2022, 48 2: 372-379. DOI:10.1134/S1068162022020030.
[3] JUN YAN LEK. Understanding the Effect of Surface Chemistry on Charge Generation and Transport in Poly (3-hexylthiophene)/CdSe Hybrid Solar Cells[J]. ACS Applied Materials & Interfaces, 2011, 3 2: 287-292. DOI:10.1021/am100938f.
[4] SANDEEP KUMAR. Efficient synthesis of piperazine-2,6-dione and 4-(1H-indole-2-carbonyl)piperazine-2,6-dione derivatives and their evaluation for anticancer activity[J]. Medicinal Chemistry Research, 2013, 22 10: 4600-4609. DOI:10.1007/s00044-012-0438-7.
Properties of Thiophene-2-ethylamine
| Melting point: | 202 °C |
| Boiling point: | 200-201 °C/750 mmHg (lit.) |
| Density | 1.087 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 190 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO, Methanol |
| form | Liquid |
| pka | 9.47±0.10(Predicted) |
| Specific Gravity | 1.087 |
| color | Colorless to yellow |
| Sensitive | Air Sensitive |
| BRN | 106962 |
| CAS DataBase Reference | 30433-91-1(CAS DataBase Reference) |
Safety information for Thiophene-2-ethylamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Thiophene-2-ethylamine
| InChIKey | HVLUYXIJZLDNIS-UHFFFAOYSA-N |
Thiophene-2-ethylamine manufacturer
JSK Chemicals
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 30433-91-1 99%View Details
30433-91-1 99%View Details
30433-91-1 -
 30433-91-1 2-Thiopheneethylamine, 96% 99%View Details
30433-91-1 2-Thiopheneethylamine, 96% 99%View Details
30433-91-1 -
 2-Thiopheneethylamine, 96% CAS 30433-91-1View Details
2-Thiopheneethylamine, 96% CAS 30433-91-1View Details
30433-91-1 -
 2-(2-Aminoethyl)thiophene CAS 30433-91-1View Details
2-(2-Aminoethyl)thiophene CAS 30433-91-1View Details
30433-91-1 -
 Thiophene-2-ethylamine 98% CAS 30433-91-1View Details
Thiophene-2-ethylamine 98% CAS 30433-91-1View Details
30433-91-1 -
 2-Thiopheneethylamine CAS 30433-91-1View Details
2-Thiopheneethylamine CAS 30433-91-1View Details
30433-91-1 -
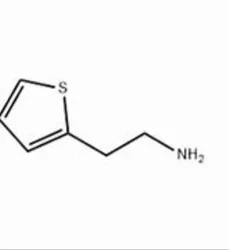 2-thiophene ethylamineView Details
2-thiophene ethylamineView Details
30433-91-1 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
