QUISQUALIC ACID
Synonym(s):β-(3,5-Dioxo-1,2,4-oxadiazolidin-2-yl)-L -alanine;3-(3,5-Dioxo-1,2,4-oxadiazolidin-2-yl)-L -alanine
- CAS NO.:52809-07-1
- Empirical Formula: C5H7N3O5
- Molecular Weight: 189.13
- MDL number: MFCD00069337
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:26:36
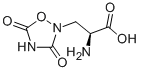
What is QUISQUALIC ACID?
Description
L-Quisqualic acid is a natural analog of glutamate that acts as an agonist at AMPA-selective and metabotropic glutamate receptors (EC50s = 170, 10, 40, and 29 nM at GluR-A, mGluR1, mGluR3, and mGluR5, respectively), as well as the ionotropic kainate receptor (GRIK4; Ki = 6.43 nM). L-Quisqualic acid is used to study receptor dynamics and as an excitotoxin to selectively destroy neurons in the brain or spinal cord.
Chemical properties
Off-White Solid
The Uses of QUISQUALIC ACID
Quisqualic acid is used to differentiate between mGluR-1 and mGluR-4. L-Quisqualic acid is an activator of GluR.
The Uses of QUISQUALIC ACID
An amino acid that is able to function as an agonist at multiple excitatory amino acid receptor substrates in the central nervous system. It also has high affinity for the kainate, AMPA and the metabotropic receptors. Inhibits the Ca2+/Cl-dependent glutamic acid uptake system in brain synaptic plasma membrane preparations.
The Uses of QUISQUALIC ACID
Quisqualic acid has been used to test ligand-gated receptors in spiral ganglion neurons.
What are the applications of Application
L-Quisqualic acid is an agonist for group I mGluR
Definition
ChEBI: Quisqualic acid is a non-proteinogenic alpha-amino acid.
General Description
Quisqualate/ Quisqualic acid is obtained from the fruits and seeds of Quisqualis chinensis. It is an agonist at two subsets of excitatory amino acid receptors metabotropic receptors that indirectly mediate calcium mobilization from intracellular stores and, ionotropic receptors that directly control membrane channels. L-quisqualic acid is an agonist of the neurotransmitter L-glutamate.
Biological Activity
Glutamate receptor agonist acting at AMPA receptors and metabotropic glutamate receptors positively linked to phosphoinositide hydrolysis. Sensitizes neurons in hippocampus to depolarization by L-AP6 (the so called 'quis' effect). Also available as part of the Group I mGlu Receptor Tocriset™ .
Biochem/physiol Actions
Active enantiomer of quisqualic acid; excitatory amino acid at glutamate receptors.
Storage
Store at RT
Purification Methods
It has been purified by ion-exchange chromatography on Dowex 50W (x 8, H+ form); the desired fractions are lyophilised and recrystallised from H2O/EtOH. It has IR (KBr) : 3400-2750br, 1830s, 1775s, 1745s and 1605s cm-1;maxand 1H NMR (NaOD/D2O, pH 13) : 3.55-3.57 (1H m, X of ABX, H-2), 3.72-3.85 (2H, AB of ABX, H-3), 1 3C NMR (D2O) : 50.1t, 53.4d, 154.8s, 159.7s and 171.3s. [Baldwin et al. J Chem Soc, Chem Commun 256 1985.] It is a quasiqualate receptor agonist [Joels et al. Proc Natl Acad Sci USA 86 3404 1989].
Properties of QUISQUALIC ACID
| Melting point: | 185-187°C dec. |
| Boiling point: | 324.36°C (rough estimate) |
| alpha | D20 +17.0° (c = 2.0 in 6M HCl) |
| Density | 1.6649 (rough estimate) |
| refractive index | 1.6190 (estimate) |
| storage temp. | 2-8°C |
| solubility | NH4OH 1 M: 20 mg/mL, clear, colorless |
| form | powder |
| pka | 2.12±0.10(Predicted) |
| color | white to off-white |
| Water Solubility | Soluble in ethanol, water. Insoluble in organic solvents. |
| Merck | 13,8177 |
| BRN | 1078734 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
Safety information for QUISQUALIC ACID
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P321:Specific treatment (see … on this label). P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for QUISQUALIC ACID
New Products
N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 1,4-bis(methylsulfonyl)butane 4-Cyano-N-Methacryloyl-3-Trifluoromethyl Aniline 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 3-Hydroxypropionitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Quisqualic acid CAS 52809-07-1View Details
Quisqualic acid CAS 52809-07-1View Details
52809-07-1 -
![6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%View Details
6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%View Details
2115779-15-0 -
 4-bromo-1H-pyrazole-3-carboxylic acid 98%View Details
4-bromo-1H-pyrazole-3-carboxylic acid 98%View Details
13745-17-0 -
 230971-72-9 (1-((2'-(2-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-72-9 (1-((2'-(2-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-72-9 -
 Isopropyl amine Hydrochloride 99%View Details
Isopropyl amine Hydrochloride 99%View Details
15572-56-2 -
 19501-58-7 99%View Details
19501-58-7 99%View Details
19501-58-7 -
 Cyclopropane-1,1-dicarboxylic acid 99%View Details
Cyclopropane-1,1-dicarboxylic acid 99%View Details
598-10-7 -
 230971-71-8 (1-((2'-(1-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-71-8 (1-((2'-(1-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-71-8
