L-AP4
Synonym(s):L -AP-4;Cartilage-Derived Morphogenetic Protein-1;CDMP-1;LAP4;L-AP4
- CAS NO.:23052-81-5
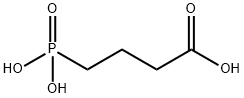
- Empirical Formula: C4H10NO5P
- Molecular Weight: 183.1
- MDL number: MFCD00083244
- EINECS: 200-258-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:12:30
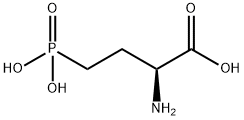
What is L-AP4?
Description
Metabotropic glutamate receptors (mGluR) function to modulate excitatory synaptic transmission in the brain. Eight subtypes (1-
Chemical properties
white to off-white crystalline powder
The Uses of L-AP4
Metabotropic glutamate receptors (mGluR) function to modulate excitatory synaptic transmission in the brain. Eight subtypes (1-8) and multiple splice variants of the mGluR have been identified and grouped based on their pharmacological properties. Group I mGluRs (subtypes 1 and 5) activate the phosphatidyl inositol pathway, while Group II (2 and 3) and Group III (4, 6, 7, and 8) inhibit adenylyl cyclase. L-AP4, an analog of L-glutamic acid, is a selective Group III mGluR agonist that functions presynaptically to suppress glutamate release (IC50 = 2.5 μM). L-AP4 has been shown to depress synaptic transmission in glutamatergic pathways in the hippocampus, olfactory bulb, and retina as well as act as an agonist at the quisqualate-sensitized AP6 site in hippocampus.
The Uses of L-AP4
L(+)-2-Amino-4-phosphonobutanoic acid (L-AP4) is a mGluR-4/6 receptor agonist.
What are the applications of Application
L(+)-2-Amino-4-phosphonobutanoic acid (L-AP4) is a mGluR-4/6 receptor agonist
Definition
ChEBI: (2S)-2-amino-4-phosphonobutanoic acid is a non-proteinogenc L-alpha-amino acid that is L-alpha-aminobutyric acid in which one of the hydrogens of the terminal methyl group has been replaced by a dihydroxy(oxido)-lambda(5)-phosphanyl group. It is a potent and selective agonist for the group III metabotropic glutamate receptors (mGluR4/6/7/8). It has a role as a metabotropic glutamate receptor agonist. It is a non-proteinogenic L-alpha-amino acid and a member of phosphonic acids.
Biological Activity
Selective group III metabotropic glutamate receptor agonist. Synaptic depressant. Agonist at the quisqualate-sensitized AP6 site in hippocampus. Also available as part of the Group III mGlu Receptor Tocriset™ and Mixed mGlu Receptor Tocriset™ .
Storage
Room temperature
Properties of L-AP4
| Melting point: | 207-215 °C |
| Boiling point: | 491.7±55.0 °C(Predicted) |
| Density | 1.628±0.06 g/cm3(Predicted) |
| storage temp. | 0-6°C |
| solubility | H2O : 50 mg/mL (273.07 mM; Need ultrasonic) |
| pka | 2.19±0.10(Predicted) |
| form | solid |
| color | White |
| Water Solubility | Soluble to 5 mM in water and to 100 mM in 1eq. NaOH |
| Sensitive | Light Sensitive |
| CAS DataBase Reference | 23052-81-5(CAS DataBase Reference) |
Safety information for L-AP4
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for L-AP4
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran
![[3H]-(L)-AP4](https://img.chemicalbook.in/StructureFile/ChemBookStructure3/GIF/CB7755385.gif)



You may like
-
 L-(+)-2-Amino-4-phosphonobutyric acid CAS 23052-81-5View Details
L-(+)-2-Amino-4-phosphonobutyric acid CAS 23052-81-5View Details
23052-81-5 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
