L-CANALINE BASE
- CAS NO.:496-93-5
- Empirical Formula: C4H10N2O3
- Molecular Weight: 134.13
- MDL number: MFCD00057642
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 16:37:02
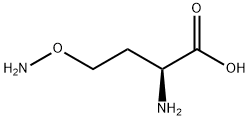
What is L-CANALINE BASE?
Description
L-Canaline is an aminooxy analog of ornithine that irreversibly inhibits aminotransferases (transaminases), including ornithine aminotransferase (Ki = 2 μM). It forms oximes with α-keto acids and aldehydes, most notably with pyridoxal phosphate, an essential cofactor of aminotransferases. L-Canaline is naturally found in plants, including legumes, and is involved in the metabolism of L-canavanine, an aminooxy analog of arginine. It is cytotoxic to a range of organisms, including bacteria, insects, and parasites.
Definition
ChEBI: L-canaline is a non-proteinogenic L-alpha-amino acid that is L-homoserine in which the hydroxy group at position 4 is substituted by an aminooxy group. It has been isolated from legumes and plays an essential role in lugume chemical defense against insects. It has a role as a plant metabolite, an antineoplastic agent, an antimetabolite and a phytogenic insecticide. It is functionally related to a L-homoserine. It is a tautomer of a L-canaline zwitterion.
Synthesis Reference(s)
Tetrahedron, 23, p. 4441, 1967 DOI: 10.1016/S0040-4020(01)88842-6
in vitro
canaline strongly inhibited the activity of pyridoxal-dependent enzymes, including amino acid decarboxylases, 5-hydroxytryptophan decarboxylase, aminotransferases, tyrosine aminotransferase, ornithine transcarbamylase and plasma diamino-oxidase. the canaline inhibition was due to complex formation between canaline and the pyridoxal coenzyme. l-canaline is one of the most potent inhibitors of pyridoxal enzymes. the ic50 value of l-canaline against ornithine aminotransferase was 3 ×10-6m [4].
in vivo
intraperitoneal administration of 500 mg of dl-canaline/kg body wt. only produced a transient inhibition of oat in brain and liver by 65-70%, suggesting that dl-canaline was not a useful tool in studies of biological consequences of oat inhibition. [1].
References
[1] bolkenius f n, kndgen b, seiler n. dl-canaline and 5-fluoromethylornithine. comparison of two inactivators of ornithine aminotransferase[j]. biochemical journal, 1990, 268(2): 409-414.
[2] rosenthal g a, rhodes d. l-canavanine transport and utilization in developing jack bean, canavalia ensiformis (l.) dc.[leguminosae][j]. plant physiology, 1984, 76(2): 541-544.
[3] peraino c, bunville l g, tahmisian t n. chemical, physical, and morphological properties of ornithine aminotransferase from rat liver[j]. journal of biological chemistry, 1969, 244(9): 2241-2249.
[4] rahiala e l, kekomki m, jnne j, et al. inhibition of pyridoxal enzymes by l-canaline[j]. biochimica et biophysica acta (bba)-enzymology, 1971, 227(2): 337-343.
Properties of L-CANALINE BASE
| Density | 1.298 |
| storage temp. | 2-8°C |
| solubility | ≤1mg/ml in DMSO |
| form | crystalline solid |
| pka | pK1: 2.40;pK2: 3.70;pK3: 9.20 (25°C) |
| color | White to off-white |
Safety information for L-CANALINE BASE
Computed Descriptors for L-CANALINE BASE
New Products
4-Bromo-2-chlorobenzonitrile 1-Chloro-4-Methyl-2-Nitrobenzene 1,3-Diethyl-1,3-Diphenylurea 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile Chlorodehydromethyl testosterone Cisplatin Prednisolone acetate Trenbolone Enanthate Benzethonium Chloride Testosterone acetate 1,3-Di Iodo Benzene Methyl 2-oxo-2,3-dihydrobenzo[d]oxazole-7-carboxylate 3-Hydroxy-4-nitrobromobenzene 4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 2-Ethyl-1,4-diaminobenzene 2-Ethylhexyl 4-aminobenzoate Boc-L-Leucinol Calcium Ascorbate Boc-Tyr-OH tert-butyl 2-oxo-1,4,9-triazaspiro[5.5]undecane-9-carboxylate 1-(1-(tert-butoxycarbonyl)piperidin-4-yl)-1H-pyrazole-4-carboxylic acid (1R,5S)-6,6-dimethylbicyclo[3.1.1]heptan-2-oneRelated products of tetrahydrofuran


You may like
-
 2124221-11-8 (4-(methylamino)phenyl)methanesulfonic acid 98%View Details
2124221-11-8 (4-(methylamino)phenyl)methanesulfonic acid 98%View Details
2124221-11-8 -
 Boc-Arg-OH HCl H2O 114622-81-0 98%View Details
Boc-Arg-OH HCl H2O 114622-81-0 98%View Details
114622-81-0 -
 2-(2-chloropyrimidin-4-yl)acetic acid 98%View Details
2-(2-chloropyrimidin-4-yl)acetic acid 98%View Details
1211581-39-3 -
 15761-38-3 Boc-Ala-OH 98%View Details
15761-38-3 Boc-Ala-OH 98%View Details
15761-38-3 -
 42074-68-0 98%View Details
42074-68-0 98%View Details
42074-68-0 -
 20866-46-0 Boc-His(Boc)-OH 98%View Details
20866-46-0 Boc-His(Boc)-OH 98%View Details
20866-46-0 -
 31954-27-5 Boc-Gly-OMe 98%View Details
31954-27-5 Boc-Gly-OMe 98%View Details
31954-27-5 -
 2-(4-(tert-butoxycarbonyl)piperazin-2-yl)acetic acid 98%View Details
2-(4-(tert-butoxycarbonyl)piperazin-2-yl)acetic acid 98%View Details
183591-72-2
