EPOXOMICIN
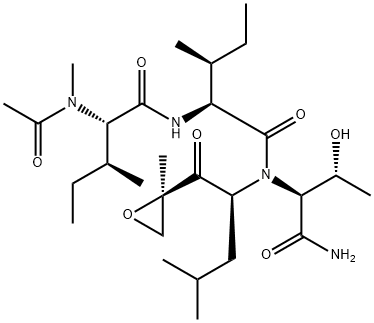
Synonym(s):N-Acetyl-N-methyl-L -isoleucyl-L -isoleucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-L -threoninamide
- CAS NO.:134381-21-8
- Empirical Formula: C28H50N4O7
- Molecular Weight: 554.72
- MDL number: MFCD03791061
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:19:24
What is EPOXOMICIN?
Description
Epoxomicin (134381-21-8) is a potent, selective and cell permeable irreversible inhibitor of the 20S proteasome.1 It does not inhibit non-proteasomal proteases such as papain, chymotrypsin, trypsin, calpain and cathepsin B at concentrations up to 50 μM.1 Epoxomicin was isolated from Actinomycete strain Q996-17 and displayed in vivo antitumor activity against B16 melanoma cells.2 Epoxomicin caused a progressive model of Parkinson’s disease in various systems.3,4,5 This model has been disputed.6,7
The Uses of EPOXOMICIN
Epoxomicin has been used:
- as an ubiquitin–proteosome system (UPS) inhibitor in pheochromocytoma PC12 cells
- as a proteasome inhibitor in mammary epithelial MCF-10A cells
- as a proteasome inhibitor in chymotryptic assay in cardiomyocytes
The Uses of EPOXOMICIN
In studies of proteasome biology.
What are the applications of Application
Epoxomicin is a potent chymotrypsin-like proteasome inhibitor (CTRL) which has also been used as an inhibitor in pheochromocytoma PC12 cells
What are the applications of Application
Epoxomicin solution is a potent CTRL (chymotrypsin-like proteasome) inhibitor
Definition
ChEBI: A tripeptide consisting of an Ile-Ile-Thr-NH2 sequence N-substituted on the threonamide amidic nitrogen with a (2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl group and with acetyl and meth l groups on the nitrogen of the isoleucine residue distal to the threonamide; a naturally occurring selective proteasome inhibitor with anti-inflammatory activity.
General Description
Epoxomicin is a linear peptide consisting of a threonine or serine residue with α′, β′-epoxyketone?derived from leucine or a γ,δ-dehydroleucine. It is a natural product isolated from?Actinomyces?sp., and is a cell-permeable, potent, selective and irreversible proteasome inhibitor.
Biochem/physiol Actions
Epoxomicin binds covalently to the catalytic subunits of proteasome. It forms an adduct with target proteins. It inhibits chymotrypsin-like activity of the proteasome. Epoxomicin also inhibits the nuclear factor κ light chain enhancer of activated B cells (NF-κB) mediated proinflammatory signalling pathway. It is also a potent antitumor and anti-inflammatory agent.
Storage
+4°C
References
1) Meng et al. (1999), Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo anti-inflammatory activity; Proc. Natl. Acad. Sci. USA, 96 10403 2) Hanada et al. (1992), Epoxomicin, a new antitumor agent of microbial origin; J. Antibiot. (Tokyo), 45 1746 3) McNaught et al. (2004), Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease; Ann. Neurol., 56 149 4) Matsui et al. (2010), Proteasome inhibition in medaka brain induces the features of Parkinson’s disease; J. Neurochem., 115 178 5) Metcalfe et al. (2012), Coordination between proteasome impairment and caspase activation leading to TAU pathology:neuroprotection by cAMP; Cell Death Diff., 3 e326 6) Kordower et al. (2006), Failure of proteasome inhibitor administration to provide a model of Parkinson’s disease in rats and monkeys; Ann. Neurol., 60 264 7) Bove et al. (2006), Proteasome inhibition and Parkinson’s disease modeling; Ann. Neurol., 60 260
Properties of EPOXOMICIN
| Melting point: | 107-109° |
| alpha | D24.5 -66.1 ± 0.4° (c = 0.5 in MeOH) |
| Boiling point: | 795.7±60.0 °C(Predicted) |
| Density | 1.117±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 10 mg/ml). |
| form | solid |
| pka | 13.02±0.46(Predicted) |
| color | White |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. |
Safety information for EPOXOMICIN
Computed Descriptors for EPOXOMICIN
| InChIKey | DOGIDQKFVLKMLQ-JTHVHQAWSA-N |
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran


You may like
-
 Epoxomicin, Synthetic CASView Details
Epoxomicin, Synthetic CASView Details -
 Epoxomicin CAS 134381-21-8View Details
Epoxomicin CAS 134381-21-8View Details
134381-21-8 -
 Epoxomicin CASView Details
Epoxomicin CASView Details -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
