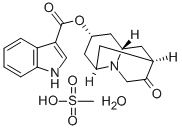
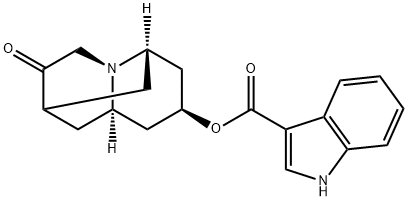
Dolasetron mesylate
Synonym(s):Anzemet hydrate;Dolasetron mesylate monohydrate;Dolasetron methanesulfonate;Dolasetron methanesulfonate hydrate
- CAS NO.:115956-13-3
- Empirical Formula: C20H26N2O7S
- Molecular Weight: 438.49
- MDL number: MFCD01718979
- EINECS: 601-404-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 19:14:41
What is Dolasetron mesylate?
Description
Dolasetron was launched as Anzemet in Australia and the US for the prevention of nausea and vomiting in chemotherapy patients. It is a highly potent and very selective antagonist of 5-HT3 receptors ; it is the sixth in this class of compounds to be marketed for the treatment of chemotherapy-induced emesis. The last two approved in this class were Nazasetron (1994) and Ramosetron (1996). Anzemet was prepared by a seven step sequence from a cyclopentenecarboxylic ester via a Robinson-Schopf cyclisation of a dialdehyde into a key 9-azabicyclo[3.3.l]nonan-3-one. In a clinical study with 164 cancer patients treated with Dolasetron mesylate prior to Cisplatin, single doses of 10- 50 mg achieved major control of nausea and emesis in 73% of subjects and were well tolerated. Results from pharmacokinetic studies in humans showed that the clinical effects and duration of action seem to be due mainly to a major plasma metabolite rapidly formed and very potent itself, the (+) enantiomeric alcohol obtained by enzymatic reduction of the cyclic ketone.
Originator
Hoechst Marion Roussel (Germany)
The Uses of Dolasetron mesylate
Dolasetron Mesylate acts as a bridged pseudopelletierine derivative; specific serotonin (5HT3) receptor antagonist. Antiemetic.
The Uses of Dolasetron mesylate
Nausea in chemotherapy;5-HT3 antagonist
The Uses of Dolasetron mesylate
Antidepressant
brand name
Anzemet (Sanofi Aventis.
Veterinary Drugs and Treatments
Dolasetron may be effective in treating severe nausea and vomiting in dogs and cats, particularly if caused by cancer chemotherapy drugs. Because it is given once a day, the injectable form of dolasetron is often preferred over ondansetron, a similarly effective antiemetic. However, for oral use in small animals, dolasetron tablets are too large (50 and 100 mg) to be practically administered.
Properties of Dolasetron mesylate
| Melting point: | 278° |
| storage temp. | Sealed in dry,2-8°C |
| solubility | H2O: >30mg/mL |
| form | powder |
| color | white to off-white |
| CAS DataBase Reference | 115956-13-3(CAS DataBase Reference) |
Safety information for Dolasetron mesylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dolasetron mesylate
Dolasetron mesylate manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

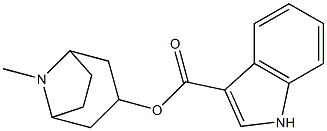
You may like
-
 Dolasetron mesylate 98%View Details
Dolasetron mesylate 98%View Details -
 115956-13-3 98%View Details
115956-13-3 98%View Details
115956-13-3 -
 Dolasetron Mesylate 99%View Details
Dolasetron Mesylate 99%View Details -
 Dolasetron mesylate hydrate CAS 115956-13-3View Details
Dolasetron mesylate hydrate CAS 115956-13-3View Details
115956-13-3 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
