Nalmefene hydrochloride
- CAS NO.:58895-64-0
- Empirical Formula: C21H26ClNO3
- Molecular Weight: 375.89
- MDL number: MFCD27937056
- EINECS: 232-572-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-06 15:14:14
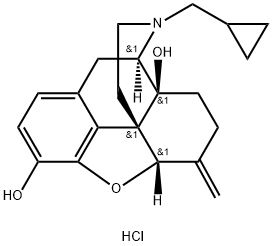
What is Nalmefene hydrochloride?
Description
Nalmefene hydrochloride has been introduced in the U.S.A. for opioid reversal following surgery, and in the reversal of opioid overdoses and epidurally administered narcotics. Nalmefene is an opioid antagonist that inhibits respiratory, analgesic and subjective effects of opioids. It has a higher potency, a longer duration of action, and superior bioavailability than the structurally related naltrexone. Nalmefene has a wide spectrum of biological activity and is in clinical trials for the treatment of interstitial cystitis, recalcitrant pruritus of cholestasis, stroke, rheumatoid arthritis, shock, CNS trauma, and alcoholism. Studies indicate that nalmefene does not induce morphine-like effects and has no apparent abuse potential.
Originator
IVAX (U.S.A.)
The Uses of Nalmefene hydrochloride
Nalmefene Hydrochloride is a structural analog of Naltrexone (N285780) with opiate antagonist activity used in pharmaceutical treatment of alcoholism. Other pharmacological applications of this compound aim to reduce food cravings, drug abuse and pulmonary disease in affected individuals. Used as an opioid-induced tranquilizer on large animals in the veterinary industry. Narcotic antagonist
What are the applications of Application
Nalmefene hydrochloride is an opioid receptor antagonist
brand name
Revex
Storage
Desiccate at RT
References
1) Osborn et al. (2010), In vivo characterization of the opioid antagonist nalmefene in mice; Life Sci., 86 624 2) Butelman et al. (2009), Unconditioned behavioral effects of the powerful kappa-opioid hallucinogen salvinorin A in non-human primates: fast onset and entry in cerebrospinal fluid; J. Pharmacol. Exp. Ther., 328 588 3) Butelman et al. (2008), The effects of herkinorin, the first mu-selective ligand from salvinorin A-derived scaffold, in neuroendocrine biomarker assay in nonhuman primates; J. Pharmacol. Exp. Ther., 327 154 4) Soyka and Rosner (2008), Opioid antagonists for pharmacological treatment of alcohol dependence – a critical review; Curr. Drug Abuse Rev., 1 280
Properties of Nalmefene hydrochloride
| Melting point: | 180-185° |
| storage temp. | Desiccate at RT |
| solubility | Soluble in Water (up to 25 mg/ml). |
| form | solid |
| pka | 7.63(at 25℃) |
| color | White |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 58895-64-0 |
Safety information for Nalmefene hydrochloride
Computed Descriptors for Nalmefene hydrochloride
Nalmefene hydrochloride manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 58895-64-0 Nalmefene hydrochloride 98%View Details
58895-64-0 Nalmefene hydrochloride 98%View Details
58895-64-0 -
 58895-64-0 98%View Details
58895-64-0 98%View Details
58895-64-0 -
 Nalmefene Hydrochloride CAS 58895-64-0View Details
Nalmefene Hydrochloride CAS 58895-64-0View Details
58895-64-0 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
