Calcium hydroxide
Synonym(s):Calcium dihydroxide;Calcium hydroxide;Carboxide
- CAS NO.:1305-62-0
- Empirical Formula: CaH2O2
- Molecular Weight: 74.09
- MDL number: MFCD00010901
- EINECS: 215-137-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-15 13:03:24
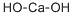
What is Calcium hydroxide ?
Description
Calcium hydroxide is a colorless crystal or white powder produced by slaking calcium oxide (quicklime) with water or by precipitating from calcium chloride and sodium hydroxide. Its natural mineral form, known as "portlandite," is relatively rare and found in volcanic, plutonic, and metamorphic rocks, as well as in burning coal dumps.
Chemical properties
Calcium hydroxide is a white powder with the chemical formula Ca(OH)?, commonly known as slaked lime or hydrated lime. It is slightly soluble in water, forms an alkaline solution with persistent basicity, and is a strong base.
Physical properties
Soft white crystalline powder; hexagonal; density 2.34 g/cm3; slightly bitter taste; loses water when heated at elevated temperatures (580°C); slightly soluble in water; Ksp 1.2x10-14; aqueous solution alkaline; soluble in glycerol and acids; insoluble in alcohol.
The Uses of Calcium hydroxide
Calcium hydroxide, a weak base, has diverse and important applications. Key uses include serving as a flocculant, in paints and sealants, mortar, Portland cement mortar, and lime plaster. Industrially, it is employed as an alkali substitute for NaOH in no-lye hair relaxers; as a depilatory agent in products like Nair; as a calcium supplement in baby formula; as a reagent for long-lasting fungicides; in reef aquariums to supply bio-available calcium and raise alkalinity; in tanning to neutralize acids, treat hides, and flocculate wastewater; in oil additive production (salicatic, sulphatic, fenatic); in manufacturing calcium stearate; in food industry water treatment (for beverages); in sugar separation from cane; processing Norwegian "Lutefisk" by softening dried cod with slaked lime and soda; producing brake pads, ebonite, and pesticides; in dentistry as an antimicrobial paste for root canals and bone regeneration; and in metal production to neutralize acidic waste gases like fluorides and chlorides before atmospheric release.
What are the applications of Application
Calcium hydroxide is a versatile alkaline compound used in food additives (e.g., processing tortillas, pickles, and sugar), as a neutralizing agent to mitigate acid rain effects, in industrial scrubbers to remove nitrogen and sulfur oxides from exhaust gases, and as a root canal filling material in dentistry.
Production Methods
Calcium hydroxide is manufactured by adding water to calcium oxide, a process called slaking.
Definition
Lime water: A solution of calcium hydroxide in water. If carbon dioxide is bubbled through lime water a milky precipitate of calcium carbonate forms. Prolonged bubbling of carbon dioxide turns the solution clear again as a result of the formation of soluble calcium hydrogencarbonate (Ca(HCO3)2).
General Description
Odorless white granules. Sinks in water.
Air & Water Reactions
Water soluble. The amount of heat generated by hydrolysis may be large.
Reactivity Profile
Nitroparaffins such as nitromethane and nitropropane form salts with inorganic bases like calcium hydroxide, and the dry salts are explosive. Chemically similar to sodium hydroxide or sodium oxide, bases neutralize acids exothermically to produce salts and water, yielding aqueous solutions with a pH greater than 7.0. Dissolving or diluting them generates significant heat. They react with metals (e.g., aluminum, zinc) to form metal oxides/hydroxides and hydrogen gas, can initiate polymerization in organic compounds (particularly epoxides), and produce flammable or toxic gases when combined with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. These materials often act as catalysts and are strong bases that form caustic solutions in water.
Hazard
Skin, eye, and upper respiratory tract irri- tant, avoid inhalation.
Health Hazard
Dust irritates eyes, nose and throat.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Calcium hydroxide is a strong alkali used as a pharmaceutical pH adjuster/buffer and antacid in topical medicinal ointments, creams, lotions, and suspensions, often in the form of lime water. It forms calcium soaps of fatty acids to produce water-in-oil emulsions (e.g., calamine liniment) and serves as a topical astringent. In cosmetics, it is a common ingredient in hair-straightening, hair-removal, and shaving products. In dentistry, it is employed as a filling agent and in dental pastes to promote secondary dentine deposition, and was traditionally used as an escharotic in Vienna Paste.
Safety Profile
Mildly toxic by ingestion. A severe eye irritant. A skin, mucous membrane, and respiratory system irritant. Mutation data reported. Causes dermatitis. Dust is considered to be a significant industrial hazard. A common air contaminant. Violent reaction with maleic anhydride, nitroethane, nitromethane, nitroparaffins, nitropropane, phosphorus. Reaction with polychloiinated phenols + potassium nitrate forms extremely toxic products. See also CALCIUM COMPOUNDS
Safety
Calcium hydroxide is used in oral and topical pharmaceutical
formulations. It is mildly toxic by ingestion. In the pure state,
calcium hydroxide is a severe skin, eye, and respiratory irritant, and
it is corrosive, causing burns. Typical exposure limits are TVL
5 mg/m3 in air.
LD50 (mouse, oral): 7.3 g/kg
LD50 (rat, oral): 7.34 g/kg
Potential Exposure
Calcium hydroxide is used in agriculture and in fertilizer manufacture; it is used in the formulation of mortar, plasters, and cements; it is used as a scrubbing and neutralizing agent in the chemical industry. For making insecticides, acaricides, and products to control arthropods
Purification Methods
Heat analytical grade calcium carbonate at 1000o during 1hour. Allow the resulting oxide to cool and add slowly to water. Heat the suspension to boiling, cool and filter through a sintered glass funnel of medium porosity (to remove soluble alkaline impurities). Dry the solid at 110o and crush it to a uniformly fine powder. [Ehrlich in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 934 1963.]
Properties of Calcium hydroxide
| Melting point: | 580 °C |
| Boiling point: | 2850 °C |
| Density | 2.24 g/mL at 25 °C (lit.) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 1.7g/l |
| form | Solid |
| pka | 12.6[at 20 ℃] |
| color | White |
| PH | 11.27(1 mM solution);12.2(10 mM solution);12.46(100 mM solution); |
| PH Range | 12.4 |
| Odor | Odorless |
| Water Solubility | 1.65 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,1673 |
| Solubility Product Constant (Ksp) | pKsp: 5.26 |
| Exposure limits | ACGIH: TWA 5 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 5 mg/m3 |
| Stability: | Stable. Incompatible with strong acids. |
| CAS DataBase Reference | 1305-62-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Calcium dihydroxide(1305-62-0) |
| EPA Substance Registry System | Calcium hydroxide (1305-62-0) |
Safety information for Calcium hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Calcium hydroxide
Calcium hydroxide manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 CALCIUM HYDROXIDE (HYDRATED LIME) 99%View Details
CALCIUM HYDROXIDE (HYDRATED LIME) 99%View Details -
 Calcium Hydroxide 98%View Details
Calcium Hydroxide 98%View Details -
 Hydrated Lime 98%View Details
Hydrated Lime 98%View Details -
 CALCIUM HYDROXIDE IP 99%View Details
CALCIUM HYDROXIDE IP 99%View Details -
 CALCIUM HYDROXIDE 99%View Details
CALCIUM HYDROXIDE 99%View Details -
 Calcium hydroxide 99%View Details
Calcium hydroxide 99%View Details -
 Calcium hydroxide 98%View Details
Calcium hydroxide 98%View Details -
 Calcium hydroxide, 95% 99%View Details
Calcium hydroxide, 95% 99%View Details
