Magnesium hydroxide
- CAS NO.:1309-42-8
- Empirical Formula: H2MgO2
- Molecular Weight: 58.32
- MDL number: MFCD00011104
- EINECS: 215-170-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-15 13:03:24
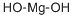
What is Magnesium hydroxide?
Absorption
About 15%-50% of magnesium hydroxide is absorbed very slowly through the small intestine.
Toxicity
LD50=8500 mg/kg (rat, oral)
Common side effects include drowsiness or flushing (warmth, redness or tingly feeling).
Daily use of magnesium hydroxide can result in fluid and electrolyte disturbances.
Excessive use of the laxative effects of magnesium hydroxide may result in abdominal cramping, nausea and/or diarrhea.
In overdose, symptoms of gastrointestinal irritation and/or watery diarrhea may occur.
Magnesium hydroxide poisoning can result in hypermagnesemia which includes symptoms of: nausea, vomiting, flushing, thirst, hypotension, drowsiness, confusion, loss of tendon reflexes, muscle weakness, respiratory depression, cardiac arrhythmias, coma and cardiac arrest.
Description
A white, bulky powder. It dissolves in dilute acids, but is practically insoluble in water and in alcohol.
Chemical properties
Magnesium hydroxide, Mg(OH)n, also known as magnesium hydrate and brucite, is a white powder that is very slightly soluble in water. It decomposes at 350°C (662 OF). It is used in the extraction of magnesium metal and as a reagent in the sulfite wood pulp process. Magnesium hydroxide is formed by the reaction of sodium hydroxide and a soluble magnesium salt solution.
Chemical properties
Magnesium hydroxide is another metal hydrate that decomposes endothermically, accompanied by the formation of water. It decomposes at 330 °C, which is 100 °C higher than alumina trihydrate, and can therefore be used in polymers that are processed at higher temperatures. Magnesium hydroxide is white, has an average particle size of 1–10 μm, density of 2.36 g/mL, refractive index of 1.58, and Mohs’ hardness of 2.00. Water loss on ignition is 31.8 wt %. Magnesium hydroxide contains 1.0 wt % Ca(OH)2 and is made by Solem Industries and Morton Thiokol.
Physical properties
Magnesium Hydroxide decomposes when heated to 360°C and forms
the oxide, MgO. It is very slightly soluble in water at
0.00122 g/100 ml and has a solubility product of
5.61×10-12. Crystals of Mg(OH)2 have a refractive
index of 1.559. Its heat of formation is –925 kJ/mol
with an entropy of 63 J/mol·K.
As a suspension in water, it is often called milk of
magnesia because of its milk-like appearance. The solid
mineral form of magnesium hydroxide is known as
“brucite”.
It can be prepared by the metathesis reaction
between any soluble magnesium salts and an alkali
hydroxide such as sodium or even ammonium:
Mg2+(aq)+2OH-(aq)→Mg(OH)2(solid)
Magnesium hydroxide is a common component of
antacids and laxatives. It interferes with the absorption
of folic acid and iron. Its low solubility makes it a weak base.
Another method of commercial manufacture of
magnesium hydroxide involves the chloride obtained from seawater. After the chloride is recrystallized
several times to obtain a level of purity, it is calcined at
about 1000°C:
MgCl2·6H2O → MgCl2+6H2O
→ Mg(OH)-xCl2-x+xHCl
→ MgO+(2-x)HCl
Occurrence
Magnesium Hydroxide occurs naturally as the mineral brucite. Brucite, usually found as a low temperature, hydrothermal vein mineral associated with calcite, aragonite, talc, or magnesite, appears as a decomposition product of magnesium silicates associated with serpentine, dolomite, magnesite, and chromite. Brucite also occurs as a hydrated form of periclase, and is found in serpentine, marble, chlorite schists, and in crystalline limestone.
The Uses of Magnesium hydroxide
Suspensions of magnesium hydroxide in water (milk
of magnesia) are used as an antacid to neutralize
stomach acid, and a laxative. Magnesium hydroxide is also used as an antiperspirant
armpit deodorant. Milk of magnesia is useful against
“canker sores” (aphthous ulcer) when used topically.
Milk of magnesia is sold for medical use to alleviate constipation,
but also to relieve indigestion and heartburn.
Magnesium hydroxide powder is used industrially as
a nonhazardous alkali to neutralize acidic wastewaters.
Solid magnesium hydroxide has also smoke suppressing
and fire retarding properties. Common uses of magnesium hydroxide as a fire retardant include plastics, roofing, and coatings. Magnesium
hydroxide is marketed as a slurry for use as a neutralizing
agent in the treatment of acidic wastewaters.
Background
Magnesium hydroxide is an inorganic compound. It is naturally found as the mineral brucite. Magnesium hydroxide can be used as an antacid or a laxative in either an oral liquid suspension or chewable tablet form. Additionally, magnesium hydroxide has smoke suppressing and flame retardant properties and is thus used commercially as a fire retardant. It can also be used topically as a deodorant or for the relief of canker sores (aphthous ulcers).
Indications
Magnesium hydroxide can be used as an antacid or a laxative depending on the administered dose.
As an antacid, it is used for the temporary relief of heartburn, upset stomach, sour stomach or acid indigestion.
As a laxative, it is used for the relief of occasional constipation by promoting bowel movements for 30 minutes and up to 6 hours.
Pharmaceutical Applications
Magnesium hydroxide [Mg(OH)2] is present in antacids because of its laxative properties and is also the main ingredient of the "milk of magnesia". The "milk of magnesia" is a suspension of Mg(OH)2 in water, which has a milk-like appearance because of the low aqueous solubility of Mg(OH)2. It is considered as a strong electrolyte and a weak base and is given to the patient for indigestion and heartburn. The alkaline suspension neutralises any excess stomach acid and therefore works as an antacid. It also stimulates intestinal movement, as the magnesium ions increases the water content in the intestines through its osmotic effect and as a result softens any faeces present.
Biochem/physiol Actions
Magnesium hydroxide is a flame retardant. Magnesium hydroxide is commonly used as an antacid which has a long-lasting effect. The liquid formulation of magnesium hydroxide is called milk of magnesia. It is used as a laxative. It is used in the treatment of diarrhea along with aluminium hydroxide. The naturally occurring form of magnesium hydroxide is brucite.
Pharmacokinetics
As an antacid, magnesium hydroxide suspension neutralizes gastric acid by reacting with hydrochloric acid in the stomach to form magnesium chloride and water. It is practically insoluble in water and does not have any effect until it reacts with the hydrochloric acid in the stomach. There, it decreases the direct acid irritant effect and increases the pH in the stomach leading to inactivation of pepsin. Magnesium hydroxide enhances the integrity of the mucosal barrier of the stomach as well as improving the tone of both the gastric and esophageal sphincters.
As a laxative, the magnesium hydroxide works by increasing the osmotic effect in the intestinal tract and drawing water in. This creates distension of the colon which results in an increase in peristaltic movement and bowel evacuation.
Safety Profile
Moderately toxic by intraperitoneal route. Human systemic effects: chlorine level changes, coma, somnolence. Incompatible with maleic anhydride, phosphorus. See also MAGNESIUM COMPOUNDS.
Veterinary Drugs and Treatments
Magnesium hydroxide in combination with aluminum salts have been used in veterinary medicine for the adjunctive treatment of esophagitis, gastric hyperacidity, peptic ulcer and gastritis. In foals and small animals, because of difficulty in administration, the frequent dosing that is often required, and availability of the histamine- 2 blocking agents (cimetidine, ranitidine, etc.), proton-pump inhibitors (e.g., omeprazole) and sucralfate, antacids have largely been relegated to adjunctive roles in therapy for these indications. Magnesium hydroxide alone (milk of magnesia) is sometimes used as an oral laxative in small animals.
Metabolism
Unless a patient is deficient in magnesium, very little is absorbed by the intestine. Overall, about 15%-50% of the magnesium hydroxide suspension is absorbed systemically. However, it does not undergo any metabolism as it is rapidly excreted in the urine.
Properties of Magnesium hydroxide
| Melting point: | 350 °C (lit.) |
| Density | 2,36 g/cm3 |
| storage temp. | Room Temperature |
| solubility | 5 M HCl: 0.1 M, clear, yellow |
| form | powder |
| color | White |
| Specific Gravity | 2.36 |
| PH Range | 9.5 - 10.5 |
| PH | 10.4(1 mM solution);10.4(10 mM solution);10.4(100 mM solution) |
| Odor | Odorless |
| Water Solubility | 0.9 mg/100 mL (18 ºC) |
| Sensitive | Air Sensitive |
| λmax | λ: 260 nm Amax: 0.030 λ: 280 nm Amax: 0.025 |
| Merck | 14,5670 |
| Solubility Product Constant (Ksp) | pKsp: 11.25 |
| Stability: | Stable. |
| CAS DataBase Reference | 1309-42-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium hydroxide(1309-42-8) |
| EPA Substance Registry System | Magnesium hydroxide (Mg(OH)2) (1309-42-8) |
Safety information for Magnesium hydroxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Magnesium hydroxide
| InChIKey | VTHJTEIRLNZDEV-UHFFFAOYSA-L |
Magnesium hydroxide manufacturer
JSK Chemicals
Sparkle Chem
Akhil Healthcare Private Limited
ASM Organics
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Magnesium Hydroxide 98%View Details
Magnesium Hydroxide 98%View Details -
 Milk of Magnesia 98%View Details
Milk of Magnesia 98%View Details -
 Magnesium Hydroxide 98%View Details
Magnesium Hydroxide 98%View Details -
 MAGNESIUM HYDROXIDE 99%View Details
MAGNESIUM HYDROXIDE 99%View Details -
 Magnesium Hydroxide 99%View Details
Magnesium Hydroxide 99%View Details -
 Magnesium hydroxide 98%View Details
Magnesium hydroxide 98%View Details -
 Magnesium hydroxide CAS 1309-42-8View Details
Magnesium hydroxide CAS 1309-42-8View Details
1309-42-8 -
 Magnesium hydroxide CAS 1309-42-8View Details
Magnesium hydroxide CAS 1309-42-8View Details
1309-42-8
