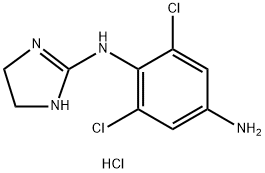
Apraclonidine hydrochloride
Synonym(s):p-Aminoclonidine monohydrochloride;2-(4-Amino-2,6-dichloroanilino)-2-imidazoline hydrochloride
- CAS NO.:73218-79-8
- Empirical Formula: C9H11Cl3N4
- Molecular Weight: 281.57
- MDL number: MFCD00135922
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-23 16:19:27
What is Apraclonidine hydrochloride?
Chemical properties
Crystalline Solid
Originator
Alfadrops,Cipla Limited,India
The Uses of Apraclonidine hydrochloride
Apraclonidine hydrochloride can be used as α-Adrenergic agonist; structural analog of clonidine and be used for treatment of post-surgical elevated intraocular pressure.
The Uses of Apraclonidine hydrochloride
a-Adrenergic agonist; structural analog of clonidine. Used for treatment of post-surgical elevated intraocular pressure
Definition
ChEBI: The hydrochloride salt of apraclonidine.
Manufacturing Process
The preparation of p-aminoclonidine (apraclonidine) consists of 6 steps.
In the first step 2,6-dichloro-4-nitroaniline was converted to 2,6-dicloro-4-
nitrophenylisothiocyanate by addition of thiophosgene in toluene according to
the method described in Great Britain Patent No.: 1,131,780 (Beck et al.).
The second step involved the conversation of 2,6-dichloro-4-nitrophenylisothiocyanate
to 1-(2-aminoethyl)-3-(2,6-dichloro-4-nitrophenyl)-thiourea
ethylenediamine solvate. The solution of 2,6-dicloro-4-nitrophenylisothiocyanate
(432 g, 1.73 mol) in 2 L of toluene was added dropwise to the
cooled (0°C) solution ethylenediamine (244 ml, 3.66 mol, 2.1 eq.) in toluene
(4 L) under a nitrogen atmosphere. 2-Propanol (1 L) was added and after 5
minutes, the solid was collected by filtration, washed with 20% 2-
propanol/toluene, and dried to a constant weight of 602 g (94%). This
product is hygroscopic, mp 120°C (dec.).
The third step was the conversation of 1-(2-aminoethyl)-3-(2,6-dichloro-4-
nitrophenyl)-thiourea ethylenediamine solvate to 2-[(2,6-dicloro-4-
nitrophenyl)imino]imidazoline ethylenediamine solvate. (500 g, 1.35 mol) of
above prepared thiourea solvate was suspended with toluene (4 L) and was
heated at reflux for 15 hours. The mixture was cooled to 23°C and 1 M
aqueous hydrochloric acid (4 L) was added. After stirring for 10 min the
biphasic mixture was filtered to remove a sticky insoluble material. The
aqueous phase was neutralized to pH=7.0 using 50% NaOH. After stirring for
1 hour the yellow solid was collected by filtration, washed with water (4 L)
and t-butyl methyl ether (2 L) and dried in air to constant weight of 195 g
(52%), m.p. 289-292°C.
The fourth step was the conversation of 2-[2,6-dichloro-4-nitrophenyl)
imino]imidazoline (150 g, 0.55 mol) in methanol (1,5 L) to 2-[(2,6-dichloro-4-
aminophenyl)imino]imidazoline by hydrogen with 30 g Raney nickel catalyst at
23°C for 22 hours. After removing the catalyst hydrogen chloride gas was
bubbled into solution until pH of the reaction mixture was 1.0. The solvent
was rotary removed in vacuum and the residual solid was slurried with 2-
propanol (1 L). The solvent was again removed by rotary evaporation, the
cream solid was triturated with 2-propanol (600 ml). After aging for 1 hour,
the solid was collected by filtration, washed with 2-propanol and t-butyl
methyl ether, and dried for 15 hours at 6°C and t-butyl methyl ether, and
dried for 15 hours at 60°C and 20 mm Hg. Yield of dihydrochloride 167 g (96%), mp 260°C (dec.).
The dihydrochloride was converted to the monochloride (step 5) by adding 5
M aqueous sodium hydroxide dropwise to pH=6.5 at 5°C for 2 hours. Yield of
hydrochloride 87%.
The last step was recrystallization of product from water. The recrystallized
material had m.p. 300°C. Calculated for: C9H10Cl2N4HCl: C, 38.39; H, 3.94;
N, 19.90; Cl, 37.78. Found: C, 38.36; H, 3.91; N, 19.83; Cl, 37.77.
brand name
Iopidine (Alcon).
Therapeutic Function
Antiglaucoma
Properties of Apraclonidine hydrochloride
| Melting point: | >2300C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 1.4 mg/mL Solutions may be stored for several days at 4°C |
| form | solid |
| color | white |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 73218-79-8(CAS DataBase Reference) |
Safety information for Apraclonidine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H370:Specific target organ toxicity, single exposure |
Computed Descriptors for Apraclonidine hydrochloride
Apraclonidine hydrochloride manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 Apraclonidine HCl 97.00% CAS 73218-79-8View Details
Apraclonidine HCl 97.00% CAS 73218-79-8View Details
73218-79-8 -
 Apraclonidine hydrochloride CAS 73218-79-8View Details
Apraclonidine hydrochloride CAS 73218-79-8View Details
73218-79-8 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
