1,1,3,3-Tetramethyldisilazane
Synonym(s):TMDS
- CAS NO.:15933-59-2
- Empirical Formula: C4H15NSi2
- Molecular Weight: 133.34
- MDL number: MFCD00025626
- EINECS: 240-072-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:25:36
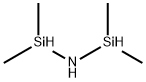
What is 1,1,3,3-Tetramethyldisilazane?
Chemical properties
Clear colorless to faintly yellow liquid
Physical properties
bp 99–100 °C; n20 D 1.4040; d 0.752 g cm?3.
The Uses of 1,1,3,3-Tetramethyldisilazane
1,1,3,3-Tetramethyldisilazane is widely used in intramolecular hydrosilation of allyl alcohols, homoallyl alcohols, and homopropargyl alcohols for the regio- and stereoselective synthesis of polyols
The Uses of 1,1,3,3-Tetramethyldisilazane
Tetramethyldisilazane is used as a gas chromatographic derivatizing reagent. Further, it reacts with phenol to prepare dimethylphenoxysilane. In addition, it is used in electronic, polymer and pharmaceutical industries.
Properties and Applications
The ICP CVD synthesis of SiCxNy: H thin films using 1,1,3,3-tetramethyldisilazane (TMDSZ ) as a single-source precursor and argon as a gas-activator[1]. Using TMDSZ as the single-source compound produced amorphous hydrogenated silicon carbonitride (a-SiCN: H) films, whereas no such films were formed when HMDSZ was used, which was attributed to the lack of Si–H bond in HMDSZ. Under the collision-free condition, the formation of ?CH3, NH3, and DMSA was demonstrated from the decomposition of TMDSZ on the heated W filament. Free-radical short-chain reactions dominate the secondary gas-phase reactions of TMDSZ in the reactor setup. The short-chain reaction is initiated by the formation of methyl radicals via the cleavage of the Si–CH3 bonds of TMDSZ. The H abstraction by the produced methyl radicals from the Si–H or the C–H bond in various stable molecules (e.g., TMDSZ) propagates the chain with a resulting radical, which recombines with another radical to terminate the chain reactions, producing a series of products in the high-mass region[2].
References
[1] Maksim N. Chagin. “Synthesis, Properties and Aging of ICP-CVD SiCxNy:H Films Formed from Tetramethyldisilazane.” Coatings (2022).
[2] James Michael Stevenson, Eric Ampong and Yujun Shi*. “Understanding the Reaction Chemistry of 1,1,3,3-Tetramethyldisilazane as a Precursor Gas in a Catalytic Chemical Vapor Deposition Process.” The Journal of Physical Chemistry A 127 44 (2023): 9185–9195.
Properties of 1,1,3,3-Tetramethyldisilazane
| Melting point: | 99-100 °C |
| Boiling point: | 99-100 °C |
| Density | 0.752 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 17 °F |
| storage temp. | Store below +30°C. |
| solubility | Miscible with common organic solvents. |
| form | clear liquid |
| pka | 9.80±0.70(Predicted) |
| Specific Gravity | 0.752 |
| color | Colorless to Almost colorless |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 741869 |
| CAS DataBase Reference | 15933-59-2(CAS DataBase Reference) |
| EPA Substance Registry System | Silanamine, N-(dimethylsilyl)-1,1-dimethyl- (15933-59-2) |
Safety information for 1,1,3,3-Tetramethyldisilazane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,1,3,3-Tetramethyldisilazane
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 1,1,3,3-Tetramethyldisilazane CAS 15933-59-2View Details
1,1,3,3-Tetramethyldisilazane CAS 15933-59-2View Details
15933-59-2 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
