Zifrosilone
- CAS NO.:132236-18-1
- Empirical Formula: C11H13F3OSi
- Molecular Weight: 246.3
- MDL number: MFCD00864135
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-05 11:31:08
What is Zifrosilone?
Originator
Zifrosilone,Marion Merrell Dow
The Uses of Zifrosilone
Inhibitor (acetylcholinesterase).
Manufacturing Process
3-Trimethylsilyl-bromobenzene:
A mixture of 1,3-dibromobenzene (25.0 g, 106.4 mmol) and
trimethylsilylchloride (11.6 g, 106.4 mmol) in diethyl ether (50 ml) was added
dropwise in 1.5 hours on magnesium (2.59 g, 106.4 mmol) in diethyl ether
(25 ml). Then the mixture was refluxed for 18 hours, cooled to 0°C, treated
with 4 N HCl (75 ml). The organic layer was separated, washed with water,
brine, dried over MgSO 4 and concentrated. 3-Trimethylsilylbromobenzene was
obtained by fractional distillation as a colorless oil (13.4 g, 55% yield, b.p.:
55-62°C/0.8 mmHg.
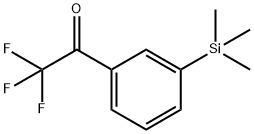
2,2,2-Trifluoro-1-(3-trimethylsilylphenyl)ethanone:
To a solution of 3-trimethylsilylbromobenzene (7.62 g, 33.3 mmol) in diethyl
ether (35 ml) was added 1.5 M n-butyl lithium in hexane (22.2 ml, 33.3
mmol) at 0°C over 10 min. Then the mixture was allowed to react 15 min at
room temperature, cooled to -78°C and ethyl trifluoroacetate (14.2 g, 100
mmol) was added over 5 min. Then the mixture was allowed to react 15 min
at -78°C, the cooling bath was removed and when the temperature rose to
0°C. 3 N HCl (35 ml) was added dropwise. The organic layer was separated,
washed with water, brine, dried over MgSO 4 and concentrated.
Chromatography (silica gel, cyclohexane/diethyl ether: 90/10) followed by
distillation under reduced pressure afforded the expected compound
(zifrosilone) as a colorless oil (4.32 g, 53% yield), boiling point 120°C/0.8 mm
Hg; Rf: 0.28 (cyclohexane/diethyl ether: 95/5).
Therapeutic Function
Acetylcholinesterase inhibitor
Properties of Zifrosilone
| Boiling point: | 215.3±35.0 °C(Predicted) |
| Density | 1.11±0.1 g/cm3(Predicted) |
Safety information for Zifrosilone
Computed Descriptors for Zifrosilone
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5