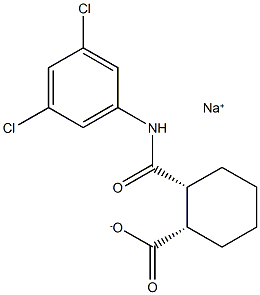
VU0155041 sodium salt
- CAS NO.:1259372-69-4
- Empirical Formula: C14H14Cl2NNaO3
- Molecular Weight: 338.162
- Update Date: 2026-01-05 11:31:08
What is VU0155041 sodium salt?
Description
VU0155041 is a positive allosteric modulator of metabotropic glutamate receptor 4 (mGluR4) with an EC50 value of 2.5 μM for glutamate response in a thallium flux assay. It is selective for mGluR4, exhibiting no effect on radioligand binding at 67 G protein-coupled receptors, ion channels, and transporters, and lacking antagonist activity at NMDA receptors in striatal medium spiny neurons at a concentration of 10 μM. In vivo, VU0155041 decreases catalepsy induced by haloperidol and reverses askinesias induced by reserpine in rat models of Parkinson’s disease. Chronic treatment with VU0155041 restores social behavior in the Oprm-/- mouse model of autism. It also increases the percentage of time spent in the open arms of the elevated plus maze, a measure of decreased anxiety, in mice.
The Uses of VU0155041 sodium salt
VU 0155041 Sodium Salt is a positive allosteric modulator of group III mGlu4 receptor that provides functional neuprotection in 6-hydroxydopamine rat models with parkinson’s disease.
What are the applications of Application
VU 0155041 sodium salt is a potent, positive allosteric mGluR-4 modulator
Properties of VU0155041 sodium salt
| storage temp. | Desiccate at RT |
| solubility | DMSO: 1 mg/ml; PBS (pH 7.2): 2 mg/ml |
| form | Powder |
| Water Solubility | Soluble to 25 mM in water |
Safety information for VU0155041 sodium salt
Computed Descriptors for VU0155041 sodium salt
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideYou may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
