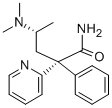
VAMICAMIDE
- CAS NO.:132373-81-0
- Empirical Formula: C18H23N3O
- Molecular Weight: 297.39
- MDL number: MFCD00909139
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-18 19:20:56
What is VAMICAMIDE?
Originator
Vamicamide,Fujisawa Pharmaceutical
The Uses of VAMICAMIDE
Antiepileptic; anticonvulsant.
Manufacturing Process
Conc. sulfuric acid (11 ml) was added to 4-(N,N-dimethylamino)-2-phenyl-2- (2-pyridyl)-valeronitrile (6.3 g) at 0°C. Then water (1 ml) was added thereto. After being heated at 90°C for 3 hours, the mixture was poured into ice-water, adjusted to pH 10 with 10% aqueous sodium hydroxide and extracted with ethyl acetate (3x50ml). The extracts were combined, and washed with water, dried over magnesium sulfate and evaporated under reduced pressure. The obtained crude crystal was recrystallized from diethyl ether to give 4-(N,N_x0002_dimethylamino)-2-phenyl-2-(2-pyridyl)-valeramide (1.89 g). MP: 132-134°C. Recrystallization from 40% water ethanol gave MP: 156-157°C. The structure of the product was confirmed by UR, NMR (CDCl3) spectra and elemental analysis.
Therapeutic Function
Anticholinergic, Spasmolytic
Properties of VAMICAMIDE
| Melting point: | 156-157° |
Safety information for VAMICAMIDE
Computed Descriptors for VAMICAMIDE
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1