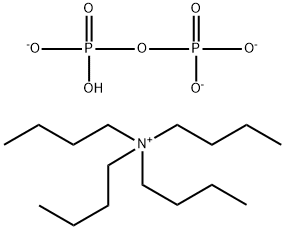
Tributylammonium pyrophosphate
- CAS NO.:5975-18-8
- Empirical Formula: C12H31NO7P2
- Molecular Weight: 363.33
- MDL number: MFCD00136019
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:26:35
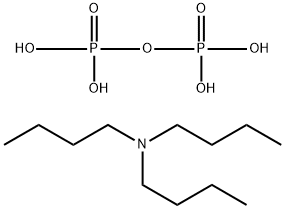
What is Tributylammonium pyrophosphate?
Description
Tributylammonium pyrophosphate is a phosphorylating reagent used in the synthesis of phosphorylated nucleosides, particularly nucleoside triphosphates used as building blocks for the synthesis of DNA, RNA and sugar nucleotides; as well as a source of cellular energy. Nucleoside triphosphates are typically synthesised by treating free nucleosides with phosphorus oxychloride, then adding tributylammonium pyrophosphate to the reaction intermediate, where the lipophilic tributylammonium salt enhances solubility in organic solvents. Additionally, tributylammonium pyrophosphate was used in the preparation of structurally complex dinucleotide-5’-triphosphates.
The Uses of Tributylammonium pyrophosphate
Bis(tributylammonium) Pyrophosphate is an phosphorylating reagent used in the synthesis of phosphorylated deoxynucleosides.
Reactant for:
Enzymic synthesis of 3′-deoxy-apio-nucleic acid duplexes
Synthesis of deoxynucleoside S-methylphosphonic acids
Preparation of fluorescent non-nucleotide ATP analog N-methylanthraniloylamideethyl triphosphate
Useful in triphosphate synthesis because of its low water content and high solubility in organic solvents.
The Uses of Tributylammonium pyrophosphate
Reactant for:
- Enzymic synthesis of 3′-deoxy-apio-nucleic acid duplexes
- Synthesis of deoxynucleoside S-methylphosphonic acids
- Preparation of fluorescent non-nucleotide ATP analog N-methylanthraniloylamideethyl triphosphate
What are the applications of Application
Tributylammonium pyrophosphate is a tributylammonium preparation
Preparation
A solution of tetrasodium pyrophosphate (3.5 g, 7.5 mmol) in water (50 mL) was eluted through a cation exchange resin (BIO-RAD AG 50 W-X8 Resin, 50–100 mesh, hydrogen form) at 4 °C. The eluent with pH of less than 3 (pH paper) was collected directly into a vigorously stirred flask containing tributylamine (2 mL, 8 mmol, 4 °C). The obtained tributylammonium pyrophosphate was dried through repeated rotary evaporation with dry MeOH and then dissolved in dry DMF (10 mL).
General Description
Tributylammonium pyrophosphate is a phosphorylating reagent used in the synthesis of modified dNTPs (deoxynucleoside triphosphates) which are important building blocks for the generation of DNA, RNA, functionalized nucleic acids.
References
Santner et al (2012). The synthesis of 2′-methylseleno adenosine and guanosine 5′-triphosphates. Bioorganic and Medicinal Chemistry 20:2416.
Hollenstein (2012). Nucleoside Triphosphates — Building Blocks for the Modification of Nucleic Acids. Molecules 17:13569-13591.
bramova et al (2007). A facile and effective synthesis of dinucleotide 5′-triphosphates. Bioorganic and Medicinal Chemistry 15:6549-6555.
Properties of Tributylammonium pyrophosphate
| Melting point: | 78 - 83°C |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C12H27N.H4O7P2/c1-4-7-10-13(11-8-5-2)12-9-6-3;1-8(2,3)7-9(4,5)6/h4-12H2,1-3H3;(H2,1,2,3)(H2,4,5,6) |
Safety information for Tributylammonium pyrophosphate
Computed Descriptors for Tributylammonium pyrophosphate
| InChIKey | WJFREWBMJPXLBS-UHFFFAOYSA-N |
| SMILES | N(CCCC)(CCCC)CCCC.O(P(O)(O)=O)P(O)(O)=O |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

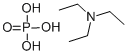
You may like
-
 5975-18-8 Bis(tributylammonium) pyrophosphate 99%View Details
5975-18-8 Bis(tributylammonium) pyrophosphate 99%View Details
5975-18-8 -
 Bis(tributylammonium) pyrophosphate 5975-18-8 98%View Details
Bis(tributylammonium) pyrophosphate 5975-18-8 98%View Details
5975-18-8 -
 Tributylammonium pyrophosphate 98% CAS 5975-18-8View Details
Tributylammonium pyrophosphate 98% CAS 5975-18-8View Details
5975-18-8 -
 Tributylammonium pyrophosphate CAS 5975-18-8View Details
Tributylammonium pyrophosphate CAS 5975-18-8View Details
5975-18-8 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
