Salidroside
Synonym(s):p-Hydroxyphenethyl glucopyranoside;2-(4-Hydroxyphenyl)ethyl β−D -glucopyranoside;Rhodioloside;Rhodosin;Tyrosol a-(β-D -glucopyranoside)
- CAS NO.:10338-51-9
- Empirical Formula: C14H20O7
- Molecular Weight: 300.3
- MDL number: MFCD00210553
- EINECS: 695-621-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-30 18:09:56
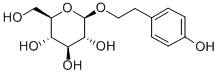
What is Salidroside?
Chemical properties
White to off-white solid
The Uses of Salidroside
Salidroside is a phenylpropanoid glycoside that exhibits a wide range of pharmacological effects. This include protective effects against neuronal death which can be applied to the treatment of Alzheimer’s disease. Cardioprotecticve, countering oxidative stress in cardiac related malignanied=v
Definition
ChEBI: Salidroside is a glycoside.
Benefits
Salidroside is the main active ingredient extracted from Rhodiola rosea and has antioxidant, anti-inflammatory, neuroprotective activities, activation of tumour suppressor kinases and other activities. It can be used in the treatment of neurological disorders (e.g. Alzheimer's disease, Parkinson's disease, traumatic brain injury, stroke, depression), cardiovascular diseases, tumours and other diseases. It is also used in the treatment of ulcerative colitis. In innate immunity, upregulated TREM1 pyroptosis-related proteins in inflamed colons were inhibited by salidroside administration further experiments in vitro showed that salidroside suppressed LPS/ATP-induced bone marrow-derived macrophages (BMDMs) pyroptosis evident by decline LDH IL-1β release as well as protein level NLRP3, caspase-1, GSDMD p30.
Mechanism of action
The salidroside present in R. rosea exerts its antistress, adaptogenic, and anti-depressive activity through stimulation of neuropeptide-Y with concomitant lower levels of HSP-72 (heat shock protein) release in brain microglial cells, ultimately modulating the HPA axis stress response. Salidroside could restore the balance of Th17/Treg cells, disrupted upon exposure to ischemia, thereby maintaining the peripheral neuron's stability after ischemia. Another neuroprotection mechanism by salidroside is maintaining monoamine neurotransmitters in the dopaminergic system. Monoamine oxidase (MAO) is an enzyme rich in the striatum and hypothalamus that could catalyze the oxidation of monoamines, such as dopamine, serotonin, adrenaline, non-adrenaline, and histamine[1-2].
References
[1] Mukherjee, Dhrubojyoti . "Role of Stress in Diseases and Its Remedial Approach by Herbal and Natural Products in Stress-Related Disease Management." 2018.
[2] Jingxuan Han. "Therapeutic potential and molecular mechanisms of salidroside in ischemic diseases." Frontiers in Pharmacology (2022): 974775.
Properties of Salidroside
| Melting point: | 159~160℃ |
| Boiling point: | 549.5±50.0 °C(Predicted) |
| Density | 1.46±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Methanol (Slightly), Pyridine (Slightly) |
| form | neat |
| pka | 9.98±0.15(Predicted) |
| form | Solid |
| color | Off-White |
| optical activity | [α]/D -29±2°, c = 0.5 in methanol |
| InChI | InChI=1/C14H20O7/c15-7-10-11(17)12(18)13(19)14(21-10)20-6-5-8-1-3-9(16)4-2-8/h1-4,10-19H,5-7H2/t10-,11-,12+,13-,14-/s3 |
| CAS DataBase Reference | 10338-51-9(CAS DataBase Reference) |
Safety information for Salidroside
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Salidroside
| InChIKey | ILRCGYURZSFMEG-RKQHYHRCSA-N |
| SMILES | [C@H]1(OCCC2C=CC(O)=CC=2)[C@H](O)[C@H]([C@H](O)[C@@H](CO)O1)O |&1:0,11,13,14,16,r| |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran





You may like
-
 Salidroside CASView Details
Salidroside CASView Details -
 Salidroside 98% (HPLC) CAS 10338-51-9View Details
Salidroside 98% (HPLC) CAS 10338-51-9View Details
10338-51-9 -
 Salidroside CAS 10338-51-9View Details
Salidroside CAS 10338-51-9View Details
10338-51-9 -
 Salidroside CAS 10338-51-9View Details
Salidroside CAS 10338-51-9View Details
10338-51-9 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
