Quinupristin
- CAS NO.:120138-50-3
- Empirical Formula: C53H67N9O10S
- Molecular Weight: 1022.23
- MDL number: MFCD00865105
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-05 11:31:08
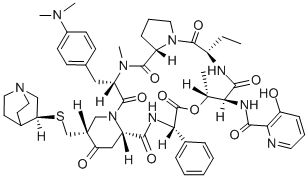
What is Quinupristin?
Description
Quinupristin is a streptogramin antibiotic that blocks peptide bond formation and peptide extension in bacteria. Quinupristin is usually combined with another streptogramin antibiotic, dalfopristin , to produce quinupristin-dalfopristin complex , known commercially as Synercid. While both quinupristin and dalfopristin have bacteriostatic effects, they act synergistically to kill Gram-positive bacteria, as well as some Gram-negative and anaerobic bacteria.
The Uses of Quinupristin
Quinupristin is a semi-synthetic analogue of virginiamycin B (ostreogyrcin B, pristinamycin IA, streptogramin B) formed by a Mannich condensation and elimination to generate an exocyclic methylene alpha to the ketone of the 4-piperidinone. Addition of quinucidinylthiol to the methylene group affords quinupristin. The structural changes provide a more hydrophobic compound with a readily ionisable group for generating a salt. Quinupristin is used commercially in synergistic combination with dalfopristin (30:70). There is little published data on the synthesis, biological or antibiotic activity of quinupristin alone, however the combination product is highly effective, including activity against antibiotic resistant strains.
The Uses of Quinupristin
Semisynthetic depsipeptide type I streptogramin. An antibacterial agent.
The Uses of Quinupristin
Quinupristin is a semi-synthetic analogue of virginiamycin B (ostreogrycin B, pristinamycin IA, streptogramin B) formed by a Mannich condensation and elimination to generate an exocyclic methylene alpha to the ketone of the 4-piperidinone. Addition of quinucidinylthiol to the methylene group affords quinupristin. The structural changes provide a more hydrophobic compound with a readily ionisable group for generating a salt. Quinupristin is used commercially in synergistic combination with dalfopristin (30:70). There is little published data on the synthesis, biological or antibiotic activity of quinupristin alone, however the combination product is highly effective, including activity against antibiotic resistant strains.
Indications
For the treatment of bacterial infections (usually in combination with dalfopristin).
Background
Quinupristin/dalfopristin is a combination of two antibiotics used to treat infections by staphylococci and by vancomycin-resistant Enterococcus faecium. Dalfopristin inhibits the early phase of protein synthesis in the bacterial ribosome and quinupristin inhibits the late phase of protein synthesis. The combination of the two components acts synergistically and is more effective in vitro than each component alone.
What are the applications of Application
Quinupristin is used commercially in synergistic combination with dalfopristin
Pharmacokinetics
Quinupristin is a streptogramin antibiotic, derived from pristinamycin I. By inhibiting the bacterial ribosomal subunits, protein synthesis is inhibited thus leading to eventual bacterial cell death or stasis.
Metabolism
Quinupristin is converted to two conjugated active major metabolites, one with glutathione and one with cysteine.
Properties of Quinupristin
| Melting point: | ~200° |
| form | Solid |
| color | White to off-white |
Safety information for Quinupristin
Computed Descriptors for Quinupristin
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

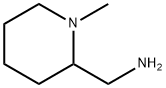

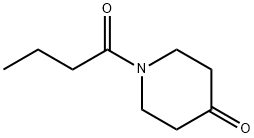
![ETHYL[(1-METHYLPIPERIDIN-2-YL)METHYL]AMINE](https://img.chemicalbook.in/CAS/GIF/66300-62-7.gif)
![Methyl[(1-methylpiperidin-2-yl)methyl]amine](https://img.chemicalbook.in/CAS/GIF/184637-50-1.gif)
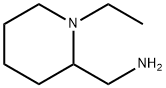
You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
