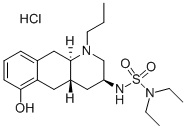
Quinagolide
- CAS NO.:87056-78-8
- Empirical Formula: C20H33N3O3S
- Molecular Weight: 395.56
- MDL number: MFCD07799967
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-17 18:22:24
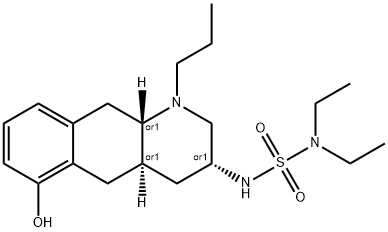
What is Quinagolide?
Absorption
The absorption of quinagolide is rapid and extensive with 95% of the dose absorbed after oral ingestion, however the absolute bioavailability is low (4 %) due to pre-systemic metabolism. The time to reach peak plasma concentration is 30-60 minutes. Prolactin-lowering effect of quinagolide at recommended therapeutic doses occurs within 2 hours after ingestion reaches a maximum within 4 to 6 hours and is maintained for at least 24 hours .
Toxicity
Most commonly observed adverse effects are nausea, vomiting, headache, dizziness and fatigue that usually appear in the beginning of initial therapy. Less frequent side effects (1 to 10%) include anorexia, abdominal pain, constipation or diarrhoea, insomnia, oedema, flushing, nasal congestion and hypotension. Orthostatic hypotension may result in faintness or syncope . Quinagolide demonstrates carcinogenic potential in animal studies but with no known relevance in humans. It is not demonstrated to be embryotoxic or teratogenic, but it is associated with reduced pregnancy rates . Oral LD50 values in mouse, rat and rabbit are 300 mg/kg, 980 mg/kg and 3200 mg/kg, respectively .
Background
Quinagolide is a non-ergot-derived selective dopamine D2 receptor agonist used for the treatment of elevated levels of prolactin or hyperprolactinaemia. Hyperprolalctinaemia is associated with gonadal dysfunction, including infertility and reduced libido, as well as long-term complications such as osteoporosis . Newer dopamine receptor agonists such as quinagolide and Cabergoline are shown to effectively inhibit prolactin secretion with improved efficacy over Bromocriptine. These drugs are effective in patients who are intolerant or resistant to Bromocriptine. Quinagolide exists as a racemate and its relevant clinical activity is mediated predominantly by the (-) enantiomer. It is typically present in the hydrochloride salt form and is marketed as oral tablets under the brand name Norprolac contained as a racemate. Quinagolide is currently available in several countries including Canada, but not approved for treatment in the United States.
Indications
Indicated for the treatment of hyperprolactinemia (idiopathic or originating from a prolactin-secreting pituitary microadenoma or macroadenoma).
Definition
ChEBI: Quinagolide is an organonitrogen heterocyclic compound and an organic heterotricyclic compound.
Pharmacokinetics
Quinagolide achieves long-lasting reduction in prolactin levels in a dose-proportional effect via selectively targeting D2 receptors as an agonist. It potently suppresses both basal and stimulated serum prolactin levels by exerting a strong inhibitory effect on the secretion of the anterior pituitary hormone prolactin.
Clinical Use
Hyperprolactinaemia
Metabolism
Quinagolide undergoes extensive first pass metabolism with sulfate and glucuronide conjugates being the major circulating metabolites. N-desethyl analogue is a biologically active metabolite while sulfate or glucuronide conjugates and N,N-didesethyl analogue are inactive metabolites.
Metabolism
Quinagolide is extensively metabolised. Quinagolide and its N-desethyl analogue are the biologically active but minor components. Their inactive sulphate or glucuronide conjugates represent the major circulating metabolites. Studies performed with 3 H-labelled quinagolide revealed that more than 95% of the drug is excreted as metabolites. About equal amounts of total radioactivity are found in faeces and urine.
Properties of Quinagolide
| Melting point: | 122.5-124° |
| Boiling point: | 539.1±60.0 °C(Predicted) |
| Density | 1.23±0.1 g/cm3(Predicted) |
| pka | 10.31±0.40(Predicted) |
Safety information for Quinagolide
Computed Descriptors for Quinagolide
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
