PUROMYCIN AMINONUCLEOSIDE
Synonym(s):3′-Amino-3′-deoxy-N6,N6-dimethyladenosine
- CAS NO.:58-60-6
- Empirical Formula: C12H18N6O3
- Molecular Weight: 294.31
- MDL number: MFCD00063462
- EINECS: 200-388-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-30 16:22:46
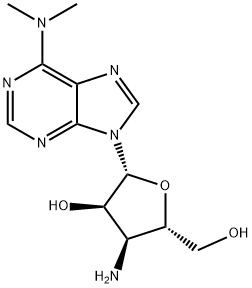
What is PUROMYCIN AMINONUCLEOSIDE?
Description
Puromycin aminonucleoside (58-60-6) is the nucleoside moiety of puromycin (Cat.# 10-2100) which does not inhibit protein synthesis or induce apoptosis.1?It acts as a glomerular epithelial cell toxin2 and as such is a useful tool for producing animal models of nephropathy3,4, or puromycin aminonucleoside nephrosis (1-2.5 mg/100g rat body weight)5. Active in vivo.
Chemical properties
White crystal
The Uses of PUROMYCIN AMINONUCLEOSIDE
Puromycin aminonucleoside is a semi-synthetic derivative of puromycin which lacks the methoxyphenylalanyl moiety. Puromycin aminonucleoside is the key intermediate in the synthesis of semi-synthetic analogues of puromycin. It does not inhibit protein synthesis or induce apoptosis, but exhibits antitumour properties. Puromycin aminonucleoside-induced nephrosis is a well-described model of human idiopathic nephrotic syndrome, suppressing integrin expression in cultured glomerular epithelial cells.
The Uses of PUROMYCIN AMINONUCLEOSIDE
Puromycin Aminonucleoside is an aminonucleoside portion of the antibiotic puromycin.
What are the applications of Application
Puromycin Aminonucleoside is a glomerular epithelial cell toxin, and aminonucleoside portion of the antibiotic puromycin.
Definition
ChEBI: Puromycin derivative that lacks the methoxyphenylalanyl group on the amine of the sugar ring.
Safety Profile
An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx,.
References
1) Chow et al. (1995), Reevaluation of the role of de novo protein synthesis in rat thymocyte apoptosis; Exp. Cell Res., 216 149 2) Krishnamurti et al. (2001), Puromycin aminonucleoside suppresses integrin expression in cultured glomerular epithelial cells; J. Am. Soc. Nephrol., 12 758 3) Egashira et al. (2006), Tryptophan-niacin metabolism in rat with puromycin aminonucleoside-induced nephrosis; Int. J. Vitam. Nutr. Res., 76 28 4) Hagiwara et al. (2006), Mitochondrial dysfunction in focal segmental glomerulosclerosis of puromycin aminonucleoside nephrosis; Kidney Int., 69 1146 5) Lowenborg et al. (2000), Glomerular function and morphology in puromycin aminonucleoside nephropathy in rats; Nephrol. Dial. Transplant, 15 1547
Properties of PUROMYCIN AMINONUCLEOSIDE
| Melting point: | 235℃ (Decomposition) |
| Boiling point: | 436.08°C (rough estimate) |
| Density | 1.2131 (rough estimate) |
| refractive index | 1.7000 (estimate) |
| Flash point: | >110°(230°F) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, slightly yellow |
| form | liquid |
| pka | 13.26±0.70(Predicted) |
| color | White |
| Water Solubility | Soluble in water at 50mg/ml. May require heating |
| BRN | 93902 |
| Stability: | Stable for 1 year as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 2 months. |
| EPA Substance Registry System | Adenosine, 3'-amino-3'-deoxy-N,N-dimethyl- (58-60-6) |
Safety information for PUROMYCIN AMINONUCLEOSIDE
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for PUROMYCIN AMINONUCLEOSIDE
| InChIKey | RYSMHWILUNYBFW-GRIPGOBMSA-N |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Puromycin aminonucleoside CAS 58-60-6View Details
Puromycin aminonucleoside CAS 58-60-6View Details
58-60-6 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
