Potassium oxalate
- CAS NO.:583-52-8
- Empirical Formula: C2K2O4
- Molecular Weight: 166.22
- MDL number: MFCD00150033
- EINECS: 209-506-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-31 14:38:37
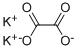
What is Potassium oxalate?
Chemical properties
Colorless, transparent crystals; odorless. Soluble in water.
Chemical properties
The anhydrous salt, mol wt 166.22, is obtained when the monohydrate is dehydrated at 160 °C. The monohydrate is preferred as a reagent in analytical chemistry and in miscellaneous uses principally because of its high solubility as compared with other simple neutral oxalates.
The Uses of Potassium oxalate
Potassium oxalate is a white crystal or powder made by neutralizing oxalic acid with potassium carbonate. It is soluble in water 1:3 but not in alcohol. Potassium oxalate was used as an early developer for gelatin plates but is best known as the developer for platinum prints.
The Uses of Potassium oxalate
Cleaning and bleaching straw, removing stains in photography; in vitro blood anticoagulant; also in analytical chemistry.
General Description
Potassium oxalate, K2C204, H20, is odorless, efforescent, water-soluble, colorless crystals that decompose when heated. Sinks in and mixes slowly with water. Used in analytical chemistry and photography, and as a bleach and oxalic acid source.
Air & Water Reactions
Water soluble.
Reactivity Profile
Potassium oxalate gives basic aqueous solutions. Reacts as a base to neutralize acids in reactions that generate heat, but less than is generated by neutralization of the bases in reactivity group 10. Can serve as a reducing agent in reactions that generate carbon dioxide.
Hazard
Toxic by inhalation and ingestion.
Health Hazard
Inhalation of dust can cause systemic poisoning. Ingestion causes burning pain in throat, esophagus, and stomach; exposed areas of mucous membrane turn white; vomiting, severe purging, weak pulse, and cardiovascular collapse may result; if death is delayed, neuromuscular symptoms develop. Contact with eyes or skin causes irritation.
Fire Hazard
Behavior in Fire: Loses water at about 160° and decomposes to carbonate with no charring. The reaction is not hazardous.
Flammability and Explosibility
Not classified
Properties of Potassium oxalate
| Density | 2.13 |
| solubility | slightly soluble in H2O |
| color | white pwd |
| Odor | odorless |
| Water Solubility | 392g/L at 20℃ |
| CAS DataBase Reference | 583-52-8(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanedioic acid, potassium salt (1:2) (583-52-8) |
Safety information for Potassium oxalate
Computed Descriptors for Potassium oxalate
Potassium oxalate manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

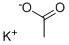
You may like
-
 583-52-8 Potassium Oxalate 99%View Details
583-52-8 Potassium Oxalate 99%View Details
583-52-8 -
 583-52-8 98%View Details
583-52-8 98%View Details
583-52-8 -
 Potassium oxalate 98%View Details
Potassium oxalate 98%View Details
583-52-8 -
 Dipotassium Oxalate Powder, 98%, 25Kg bagView Details
Dipotassium Oxalate Powder, 98%, 25Kg bagView Details
583-52-8 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
