Pilsicainide hydrochloride
Synonym(s):N-(2,6-Dimethylphenyl)tetrahydro-1H-pyrrolizine-7a(5H)-acetamide hydrochloride;Pilzicainide hydrochloride;SUN 1165
- CAS NO.:88069-49-2
- Empirical Formula: C17H24N2O
- Molecular Weight: 272.39
- MDL number: MFCD00903769
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
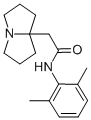
What is Pilsicainide hydrochloride?
Description
Pilsicainide hydrochloride is a new pynolizidine lidocaine derivative with antiarrhythmic activity. It is the first pharmaceutical product introduced by Suntory and was solely developed in Japan. The compound is reported to be highly effective in the treatment of premature ventricular contraction. It has no anticholinergic or CNS activity and is not as likely as other class 1C antiarrhythmics to have restricted indications.
Originator
Suntory (Japan)
The Uses of Pilsicainide hydrochloride
Pilsicainide hydrochloride has been used as a sodium channel blocker:
- to study its effects on electrophysiological parameters in guinea pig pulmonary vein preparation
- to study its effects on Ca2+?release and arrhythmic events in Andersen-Tawil syndrome induced pluripotent stem cells (ATS-iPSC)-derived cardiomyocytes
- to study its electrophysiological effects on the guinea pig atrium
What are the applications of Application
Pilsicainide hydrochloride is a pure sodium channel protein inhibitor
brand name
Sunrhythm
Biochem/physiol Actions
Pilsicainide hydrochloride is a pure sodium channel blocker and an open channel blocker of Na+1 channels. Pilsicainide was defined as a pure sodium channel blocker. The compound is classified as a class Ic antiarrhythmic drug, and was originally developed in Japan. Similar to Lidocaine, Pilsicainide binds to open channels, but slowly. Pilsicainide is capable of selectively blocking the late currents in the mutant Na(+) channels that show dominant abnormal burst openings such as in δKPQ mutants. The compound is used to induce Brugada syndrome (BS) in animal models.
Properties of Pilsicainide hydrochloride
| Melting point: | 212-214° |
| storage temp. | room temp |
| solubility | Chloroform (Slightly), Water (Slightly) |
| form | powder |
| color | white to beige |
Safety information for Pilsicainide hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Pilsicainide hydrochloride
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Pilsicainide Hydrochloride CAS 88069-49-2View Details
Pilsicainide Hydrochloride CAS 88069-49-2View Details
88069-49-2 -
 Pilsicainide hydrochloride CAS 88069-49-2View Details
Pilsicainide hydrochloride CAS 88069-49-2View Details
88069-49-2 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
