OBLIMERSEN SODIUM
- CAS NO.:190977-41-4
- Empirical Formula: C172H204N62Na17O91P17S17
- Molecular Weight: 6058.305987
- MDL number: MFCD07772392
- Update Date: 2025-10-28 16:00:23
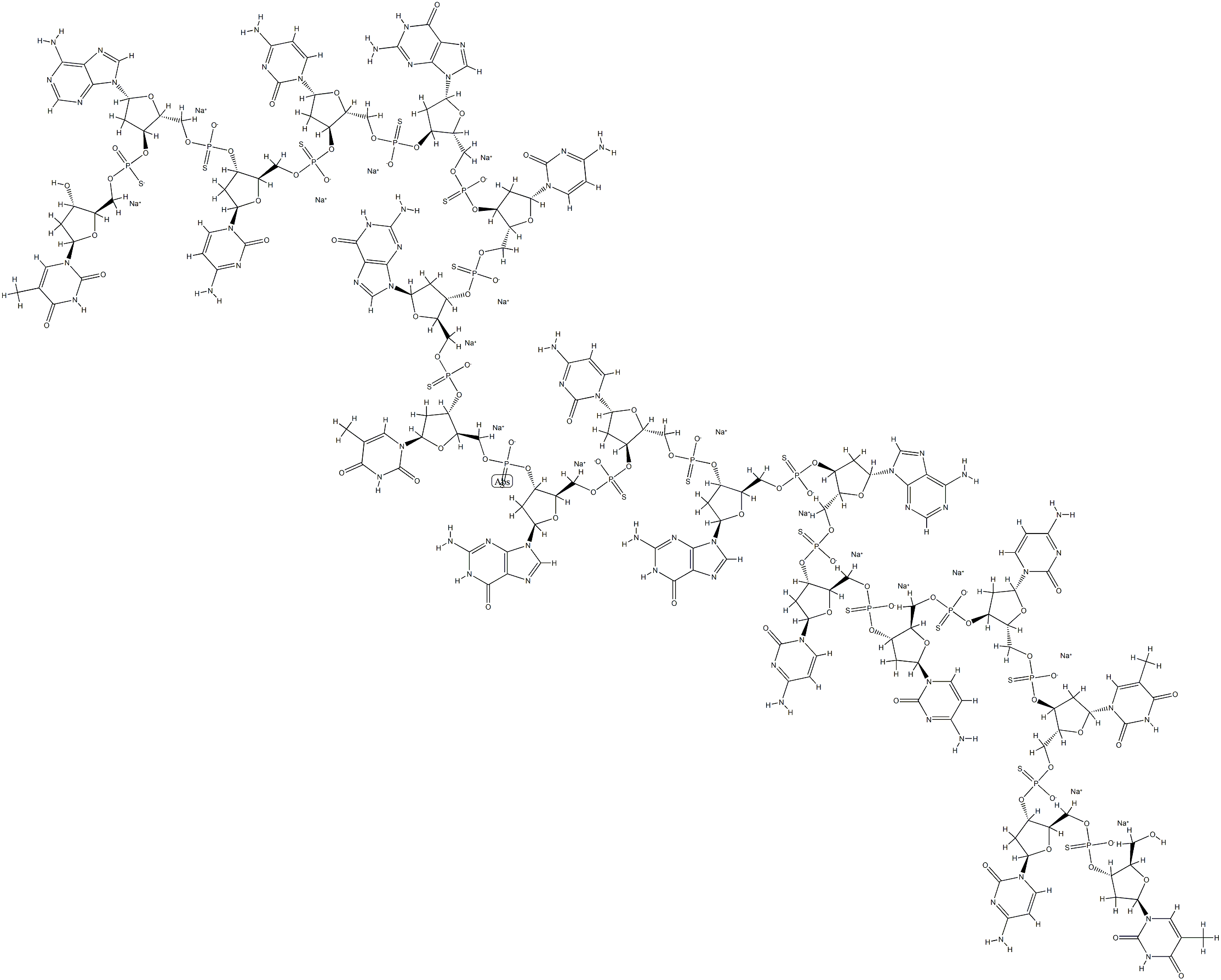
What is OBLIMERSEN SODIUM?
Description
As the representative antisense oligonucleotide, Oblimersen (G3139, Genasense) is an 18-base phosphor- othioate antisense oligodeoxynucleotide that was developed to treat CLL, B-cell lymphoma, lung, and breast cancers. Oblimersen binds with the first six codons of Bcl-2 mRNA open reading frame and mediates RNA cleavage by RNase H to downregulate Bcl-2. The antitumor effect of oblimersen is associated with both apoptotic and nonapoptotic pathways.
In the apoptotic pathway, Bcl-2 downregulation by oblimersen could increase Bax and poly (ADP-ribose) Polymerase (PARP) to release cytochrome cand Smac/DIABLO to pro- mote apoptosis. For the nonapoptotic pathway, Bcl-2 downregulation by oblimersen may have promoted the release of Beclin-1 to induce autophagic cell death. In addition, oblimersen has been shown to enhance tumor immunity by promoting polyclonal antibodies production and activating dendritic cell maturation. In monotherapy, oblimersen failed in phase-III clinical trials of treating lymphoid malignancies, which could be due to limited uptake, intracellular compartmentalization, or degradation by nuclear enzymes. On the other hand, oblimersen has been found to increase the overall survival when combined with other chemotherapeutic drugs, such as dacarbazine, fludarabine, and cyclophosphamide, by increasing the sensitivity of those drugs.
The Uses of OBLIMERSEN SODIUM
Treatment of cancer and rheumatological diseases (an antisense oligonucleotide).
brand name
Genasense (Genta).
Clinical Use
Genta is developing a phosphorothioate antisense drug called oblimersen sodium that is complementary to the first six codons of the open reading frame of the human bcl-2 mRNA sequence. Oblimersen turns off production of the apoptosis inhibitor Bcl2 in tumor cells. This appears to increase a tumor cell's sensitivity to a variety of other anticancer therapies and, ultimately, may lead to cell death.
Safety information for OBLIMERSEN SODIUM
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran






You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
