MG-132
Synonym(s):Carbobenzoxy-L-leucyl-L-leucyl-L-leucinal, Z-LLL-CHO, Proteasome Inhibitor XI;MG-132;Proteasome Inhibitor XI, Z-Leu-Leu-Leu-CHO, Carbobenzoxy-L-leucyl-L-leucyl-L-leucinal;Z-Leu-Leu-Leu-al
- CAS NO.:133407-82-6
- Empirical Formula: C26H41N3O5
- Molecular Weight: 475.62
- MDL number: MFCD00674886
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-31 10:02:37
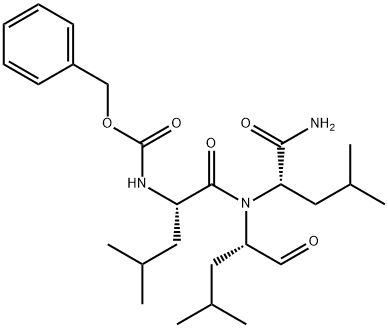
What is MG-132?
Description
MG-132 (133407-82-6) is a specific inhibitor of the chymotrypsin-like activity of the 20S proteasome (IC50=100 nM with Z-LLL-AMC as substrate).1 Also inhibits calpain (IC50=1.25 μM).1 Suppresses gastric cancer cell proliferation and induces macro-autophagy.2 Activates stress kinases and induces Hsp72.3 Induces neurite outgrowth.1 MG-132 blocks NFκB activation by blocking IκB proteolysis (IC50=3 μM).4 Cell permeable.
The Uses of MG-132
MG 132 is a potent, membrane-permeable proteasome inhibitor. It induces neurite outgrowth in PC12 cells. Neuroprotective product.
The Uses of MG-132
A proteasome and NF-κB inhibitor.
What are the applications of Application
MG-132 [Z-Leu- Leu-Leu-CHO] is a proteasome and NF-κB inhibitor which is used as a tool for perturbing the proteasome-regulated degradation of intracellular proteins
Definition
ChEBI: A tripeptide that is L-leucyl-L-leucyl-L-leucine in which the C-terminal carboxy group has been reduced to the corresponding aldehyde and the N-terminal amino group is protected as its benzyloxycarbonyl derivative.
General Description
Potent, reversible, and cell-permeable proteasome inhibitor (Ki = 4 nM). Reduces the degradation of ubiquitin-conjugated proteins in mammalian cells and permeable stains of yeast by the 26S complex without affecting its ATPase or isopeptidase activities. Activates c-Jun N-terminal kinase (JNK1) and inhibits NF-κB activation (IC50 = 3 μM).
Biological Activity
Potent cell-permeable inhibitor of proteasome (IC 50 = 100 nM) and calpain (IC 50 = 1.2 μ M). Inhibits TNF- α -induced NF- κ B activation and I κ B α degradation. Induces neurite outgrowth in PC12 cells and has anticancer properties in vitro .
Biochem/physiol Actions
Cell permeable: yes
Storage
-20°C (desiccate)
References
1) Tsubuki et al. (1996), Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine; J. Biochem.,? 119 572 2) Wu? et al. (2010), Macroautophagy and ERK phosphorylation counteract the antiproliferative effect of proteasome inhibitor in gastric cancer cells; Autophagy, 6 228 3) Meriin? et al. (1998), Proteasome inhibitors activate stress kinases and induce Hsp72. Diverse effects on apoptosis; J. Biol. Chem., 273 6373 4) Fiedler et al. (1998), Inhibition of TNF-alpha-induced NF-kappaB activation and IL-8 release in A549 cells with the proteasome inhibitor MG-132; Am. J. Respir. Cell Mol. Biol., 19 259
Properties of MG-132
| Melting point: | 80-84℃ (DEC.) |
| Boiling point: | 682.0±55.0 °C(Predicted) |
| alpha | -61~-67° |
| Density | 1.073 |
| Flash point: | 366℃ |
| storage temp. | -20°C |
| solubility | methanol: 1 mg/mL |
| form | solid film |
| pka | 11.14±0.46(Predicted) |
| color | White |
| Water Solubility | Soluble in ethanol, chloroform, methanol, water. |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO, DMF or ethanol may be stored at -20° for up to 1 week. |
Safety information for MG-132
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for MG-132
| InChIKey | TZYWCYJVHRLUCT-VABKMULXSA-N |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran







You may like
-
 Z-Leu-Leu-Leu-aldehyde 98.00% CAS 133407-82-6View Details
Z-Leu-Leu-Leu-aldehyde 98.00% CAS 133407-82-6View Details
133407-82-6 -
 MG-132 CAS 133407-82-6View Details
MG-132 CAS 133407-82-6View Details
133407-82-6 -
 MG-132, ≥95% by HPLC CAS 133407-82-6View Details
MG-132, ≥95% by HPLC CAS 133407-82-6View Details
133407-82-6 -
 Z-Leu-Leu-Leu-al CAS 133407-82-6View Details
Z-Leu-Leu-Leu-al CAS 133407-82-6View Details
133407-82-6 -
 MG-132 CAS 133407-82-6View Details
MG-132 CAS 133407-82-6View Details
133407-82-6 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
