LEVOCABASTINE
- CAS NO.:79516-68-0
- Empirical Formula: C26H29FN2O2
- Molecular Weight: 420.52
- MDL number: MFCD00672639
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-05 11:31:08
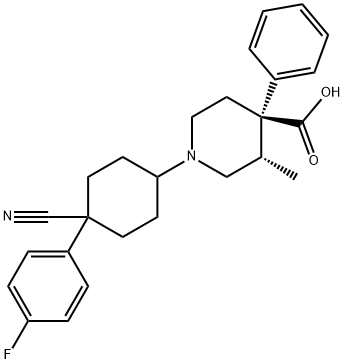
What is LEVOCABASTINE?
Absorption
After instillation in the eye, levocabastine is systemically absorbed, albeit at low levels.
Toxicity
Adverse effects include visual disturbances, dry mouth, cough, nausea, eyelid edema and lacrimation.
Originator
Livostin,Janssen-Cilag,Belgium
The Uses of LEVOCABASTINE
Anti histaminic.
Background
Levocabastine is a selective second-generation H1-receptor antagonist used for allergic conjunctivitis. Levocabastine was discovered at Janssen Pharmaceutica in 1979.
Indications
As an ophthalmic for the temporary relief of the signs and symptoms of seasonal allergic conjunctivitis. Also used as a nasal spray for allergic rhinitis.
Definition
ChEBI: Levocabastine is a member of piperidines.
Manufacturing Process
4-Cyano-4-(4-fluorophenyl)-heptanedioic acid diethyl ester is obtained by addition of ethyl acrylate to the anion from p-fluorophenylacetonitrile. By base catalyzed cyclization of these diester (sodium methoxide, 60°C, xylene) is synthesized an intermediate that after decarboethoxylation gives 1-(4- fluorophenyl)-4-oxycyclohexanecarbonitrile. By condensation of 3-methyl-5- phenylpiperidine-4-carboxylic acid benzyl ester and 1-(4-fluorophenyl)-4- oxycyclohexanecarbonitrile under reductive hydrogenation conditions (palladium-on-charcoal catalyst, 50°C, in ethanol) is prepared benzyl ester 4- piperidinecarboxylic acid, 1-(4-cyano-4-(4-fluorophenyl)cyclohexyl)-3-methyl- 4-phenyl-, (3S-(1(cis),3α,4β))-. The benzyl protecting group is then removed by hydrogenation method and 4-piperidinecarboxylic acid, 1-(4-cyano-4-(4- fluorophenyl)cyclohexyl)-3-methyl-4-phenyl-, (3S-(1(cis),3α,4β))- obtained is transformed into 4-piperidinecarboxylic acid, 1-(4-cyano-4-(4-fluorophenyl) cyclohexyl)-3-methyl-4-phenyl-, hydrochloride, (3S-(1(cis), 3α,4β))- (Levocabastine hydrochloride)
brand name
Livostin (Novartis).
Therapeutic Function
Antihistaminic
Pharmacokinetics
Levocabastine is a selective histamine H1-receptor antagonist exerting inhibitory effects on the release of chemical mediators from mast cells and on the chemotaxis of polymorphonuclear leukocytes and eosinophils. Both histamine and antigens induced conjunctivitis can be inhibited by levocabastine. Levocabastine can also reduce symptoms of allergic rhinitis by preventing an increase in vascular permeability of nasal mucosa.
Metabolism
Mostly unchanged. 10 to 20% is metabolized to the acylglucuronide of levocabastine.
Properties of LEVOCABASTINE
| Boiling point: | 589.9±50.0 °C(Predicted) |
| Density | 1.23 |
| pka | 3.57±0.40(Predicted) |
Safety information for LEVOCABASTINE
Computed Descriptors for LEVOCABASTINE
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
