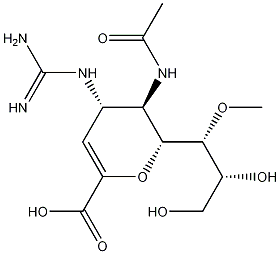
laninamivir octanoate
- CAS NO.:203120-46-1
- Empirical Formula: C21H36N4O8
- Molecular Weight: 472.53
- MDL number: MFCD28138619
- Update Date: 2026-01-21 18:05:59
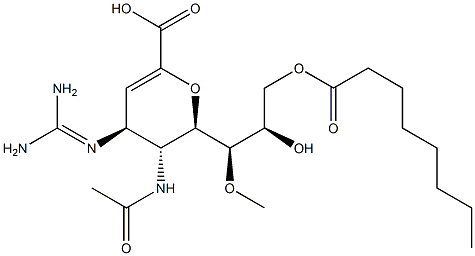
What is laninamivir octanoate?
The Uses of laninamivir octanoate
Laninamivir
The Uses of laninamivir octanoate
Laninamivir (L174000) prodrug; a novel long-acting neuraminidase inhibitor.
What are the applications of Application
Laninamivir Octanoate is a prodrug of a novel long-acting neuraminidase inhibitor
Definition
ChEBI: Laninamivir octanoate hydrate is a fatty acid ester.
Clinical Use
Laninamivir octanote, a prodrug of a potent neuraminidase inhibitor (LANI), was approved and launched in 2010 in Japan for the treatment of influenza A and influenza B. This ester prodrug of a potent neuraminidase inhibitor was designed to permeate from the lung tissue to the plasma and then hydrolyze at such a rate to reveal the active form (laninamivir) as a long-acting therapeutic agent. Neuraminidase cleaves the glycosidic linkages of neuraminic acids which are responsible for binding new viruses to infected cells, thereby allowing viruses to release and infect other cells. Neuraminidase is essential for the replication of all influenza viruses. Like other neuraminidase inhibitors, laninamivir octanoate is a sialic acid analogue which is structurally similar to zanamivir, differing only by changing one of the hydroxy groups with a methyl ether substitution on the triol side chain. Laninamivir is administered via an inhalable formulation (20 mg, dry powder inhaler) and results from clinical trials of the drug have demonstrated that a single inhaled dose is as effective as a 5-day course of oseltamivir for treatment of influenza.
Synthesis
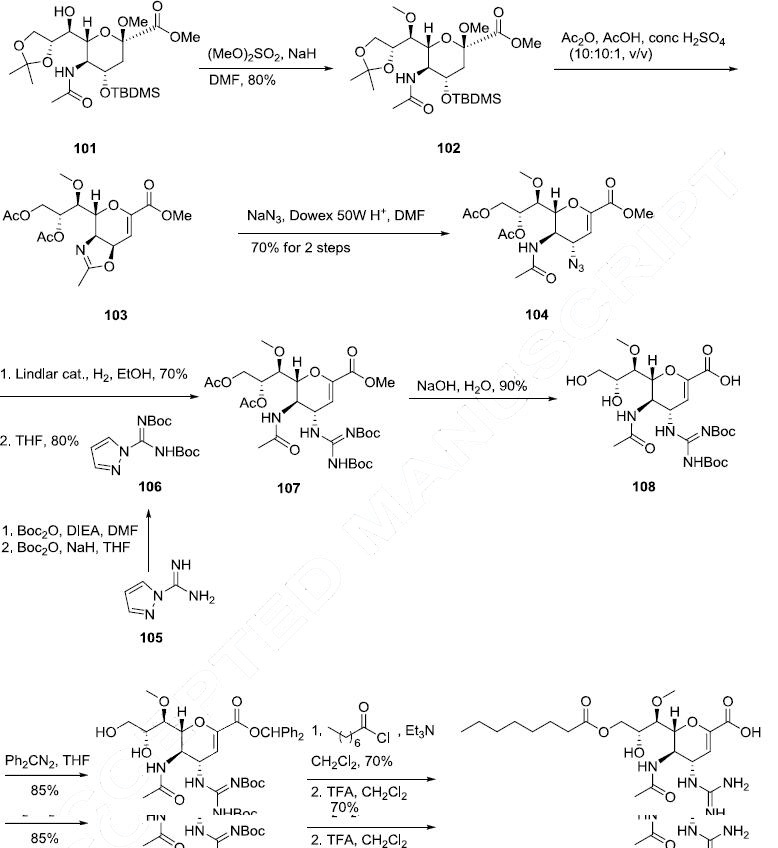
The synthesis of laninamivir octanoate began with the well-documented sugar intermediate 101. Alcohol 101 was alkylated with dimethyl sulfate in the presence of NaH in DMF to give methyl ether 102 in 80% yield. Acetonide 102 was then deprotected and subsequently acylated with Ac2O, AcOH, and H2SO4 (10:10:1, v/v) which resulted in oxazoline formation along with elimination of the methoxy functionality to furnish |�|?-unsaturated ester 103. Exposure of oxazoline 103 to NaN3 in the presence of Dowex 50W/H+ produced the transamidoazide 104 in 70% yield over two steps. Azide 104 was then subjected to guanidine formation conditions utilizing N,N-bis(tertbutoxycarbonyl)-1H-pyrazole-1-carboxyamidine (106), which was prepared from pyrazole-1-carboxamidine (105) by consecutive protection of the amidine nitrogens, first by treatment with Boc anhydride and diisopropylethyl amine (DIEA) in DMF, and then subsequent treatment to Boc anhydride in the presence of NaH in THF to give 107 in 80% yield. The protected guanidine 107 was hydrolyzed under basic conditions to give the corresponding acid 108 in good yield. Acid 108 was esterified with diphenyl diazomethane in THF to provide 109 in 85% yield. Finally, the primary alcohol within diol 109 was selectively acylated with octanoyl chloride in the presence of TEA, followed by de-protection with TFA in CH2Cl2 to give laninamivir octanote (VIII) in 70% yield.
Properties of laninamivir octanoate
| Melting point: | 224-228°C (dec.) |
| Density | 1.35 |
| storage temp. | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| solubility | DMSO (Slightly), Methanol (Very Slightly) |
| form | Solid |
| pka | 3.80±0.70(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for laninamivir octanoate
Computed Descriptors for laninamivir octanoate
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 Laninamivir octanoate CAS 203120-46-1View Details
Laninamivir octanoate CAS 203120-46-1View Details
203120-46-1 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
