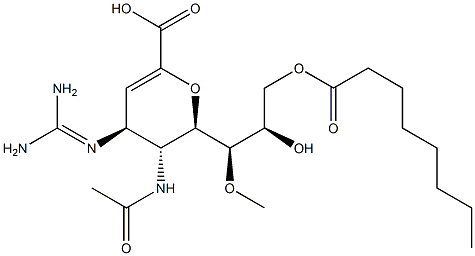
(4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid
- CAS NO.:203120-17-6
- Empirical Formula: C13H22N4O7
- Molecular Weight: 346.34
- MDL number: MFCD18071611
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 10:51:09
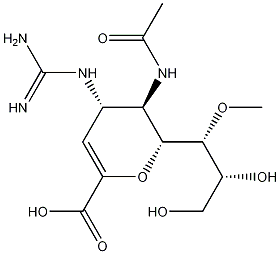
What is (4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid?
Description
Laninamivir octanoate (CS-8958), an ester prodrug form of the neuraminidase (NA) inhibitor laninamivir (R-125489), was approved in Japan in 2010 for treatment of influenza virus infections. Laninamivir octanoate is given by intranasal administration at a 20 mg or 40 mg dose. It has a long half-life in humans such that efficacy can be achieved after only a single dose. In addition to vaccines for immunoprophylaxis, antiviral drugs play an essential role in the treatment of influenza virus infections. Two viral proteins have been targeted for therapeutic intervention: the M2 ion channel and NA.
Originator
Sankyo Co., Ltd. (Japan)
The Uses of (4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid
A new potent labelled neuraminidase
The Uses of (4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid
A new potent neuraminidase (NA) inhibitor, shows long-acting anti-influenza virus activity.
What are the applications of Application
Laninamivir-d3 is a potent labelled neuraminidase (NA) inhibitor
What are the applications of Application
Laninamivir is a new potent neuraminidase (NA) inhibitor, which shows long-acting anti-influenza virus activity
Definition
ChEBI: Laninamivir is a member of acetamides.
brand name
Inavir
Synthesis
Laninamivir octanoate is prepared starting from a neuraminic acid precursor. The route from 2,3-didehydroneuramic acid entails a multistep sequence to protect the acid and hydroxyl groups at the 4-, 20-, and 30-positions. Methylation of the remaining 10-hydroxyl by treatment with dimethylsulfate and NaH is followed by conversion of the 4-hydroxyl to an amine Cleavage of the 20,30-dihydroxy protecting group, conversion of the 4- NH2 to the guanidine, and acylation of the 30-OH group afford laninamivir octanoate. This three-step sequence can be reordered such that the guanidine is introduced first, followed by deprotection of the 20,30-diOH groups and acylation. An alternative sequence involves a Boc-protected guanidine intermediate, which is converted in a four-step sequence (deprotection of the acid and 20,30-hydroxyl groups, reprotection of the acid as its diphenylmethyl ether, acylation of the 30-OH and deprotection of the guanidine group) to laninamivir octanoate. Laninamivir can also be synthesized from the a-methyl glycoside of N-acetylneuramic acid methyl ester by an analogous route.
Properties of (4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid
| Melting point: | >300°C (dec.) |
| Density | 1.61 |
| storage temp. | -20°C Freezer |
| solubility | Water (Slightly) |
| form | Solid |
| pka | 3.81±0.70(Predicted) |
| color | White to Off-White |
| Water Solubility | Water : 5 mg/mL (14.44 mM; Need ultrasonic) |
Safety information for (4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid
Computed Descriptors for (4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8