DOLOMITE
- CAS NO.:16389-88-1
- Empirical Formula: C2CaMgO6
- Molecular Weight: 184.4
- MDL number: MFCD03613593
- EINECS: 240-440-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:02

What is DOLOMITE?
Description
Dolomite is a massive calcareous sedimentary rock made of the mineral dolomite [CaMg(CO3)2, rhombohedral]. Usually dolomite as a rock contains, apart from dolomite, other carbonates (e.g., calcite, magnesite, and siderite), along with some silica and alumina, mostly as clays. For commercial purposes, the mass fraction of combined impurities must be below 7 wt.%, above which it becomes unsuitable for industrial use and is then used only for road ballasts and building stones. When the percentage of calcium carbonate (CaCO3) is above 10 wt.% or more over the theoretical composition, the rock is termed calcitic dolomite, while with a departure from theoretical magnesite content the rock is called dolomitic limestone. With variations in MgCO3 between 5 and 10 wt.%, it is called magnesian limestone, and when it contains up to 5 wt.% MgCO3 or less it is considered limestone for all purposes.
Physical properties
Habit: rhombohedral, crystalline, massive, botryoidal, globular, stalactitic.
Color: white, gray, reddish white, brownish white, or gray.
Luster: vitreous (i.e., glassy).
Diaphaneity: transparent to translucent.
Streak: white.
Cleavage: (1011) perfect.
Twinning: {0001}, {1010}, {1110}, {0112}.
Fracture: brittle, subconchoidal.
Chemical: readily dissolved by strong mineral acids with evolution of carbon dioxide.
Occurrence: sedimentary rocks.
The Uses of DOLOMITE
Pure dolomite, without calcining, is chiefly used as refractory, ramming, and felting material in steel-melting shops and as fluxing material in blast-furnace operations in secondary steel and in the production of ferromanganese. Moreover, the magnesia in dolomite acts as desulfurizating agent in molten iron metal. Generally, two grades of dolomite are used, one is called blast furnace grade and the other steel melting shop grade. Dolomite is also used to a lesser extent in the glass industry, especially in sheetglass manufacture. It also finds use in the manufacture of mineral wool. Dolomite is also a useful source for the production of magnesite by reacting calcined dolomite with seawater.
Definition
A mineral, CaCO3.MgCO3, used as an ore of magnesium and for the lining of openhearth steel furnaces and Bessemer converters.
Definition
dolomite: A carbonate mineral consistingof a mixed calcium–magnesiumcarbonate, CaCO3.MgCO3,crystallizing in the rhombohedralsystem. It is usually white or colourless.The term is also used to denotea rock with a high ratio of magnesiumto calcium carbonate. See limestone.
Agricultural Uses
Dolomite is a carbonate of calcium and magnesium. It is a mineral of formula CaMg(CO3)2 with a rhombohedral system. It has a density of 2.8 g/cc and hardness of 4 on the Mohs scale.
Dolomite has a vitreous lustre which is between transparent and translucent. It effervesces with hot concentratedh ydrochloric acid (HCl).
A carbonate-rich sedimentary rock of chemical or biological origin contains 50% or more carbonate, of which at least half is in the form of dolomite. The color of dolomite is darker than limestone and does not give any effervescence with cold and dilute HCl.
Dolomite may also contain iron and manganese. Depending on the proportion of dolomite and calcite, there are many transitional phases between calcareous dolomites and dolomitic limestones.
Dolomite has multiple applications. It is used in the manufacture of magnesium and its compounds. It is also used as building material, as a refractory for furnaces, as a fertilizer, for the removal of sulphur dioxide from stack gases and in ceramics.
Industrial uses
Dolomite is the carbonate mineral CaMg(CO3)2,often with small amounts of iron, manganese,or excess calcium, which replace some of themagnesium; cobalt, zinc, lead, and barium aremore rarely found.
Dolomite is a very common mineral, occurringin a variety of geologic settings. It is oftenfound in ultrabasic igneous rocks, notably incarbonates and serpentinites, in metamorphosedcarbonate sediments, where it may recrystallize to form dolomite marbles, and inhydrothermal veins. The primary occurrence ofdolomite is in sedimentary deposits, where itconstitutes the major component of dolomiterock and is often present in limestones.
Dolomite is normally white or colorlesswith a specific gravity of 2.9 and a hardness of3.5-4 on Mohs scale.
Properties of DOLOMITE
| Density | 2.07 g/cm3 |
| color | White |
| Dielectric constant | 6.8-8.0(0.0℃) |
| EPA Substance Registry System | Dolomite (CaMg(CO3)2) (16389-88-1) |
Safety information for DOLOMITE
Computed Descriptors for DOLOMITE
DOLOMITE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

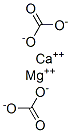



You may like
-
 Dolomite 99%View Details
Dolomite 99%View Details -
 16389-88-1 99%View Details
16389-88-1 99%View Details
16389-88-1 -
 16389-88-1 Dolomite 98%View Details
16389-88-1 Dolomite 98%View Details
16389-88-1 -
 Dolomite 16389-88-1 98%View Details
Dolomite 16389-88-1 98%View Details
16389-88-1 -
 16389-88-1 Dolomite 98%View Details
16389-88-1 Dolomite 98%View Details
16389-88-1 -
 16389-88-1 98%View Details
16389-88-1 98%View Details
16389-88-1 -
 Dolomite 16389-88-1 98%View Details
Dolomite 16389-88-1 98%View Details
16389-88-1 -
 Dolomite Powder, 97%, Industrial GradeView Details
Dolomite Powder, 97%, Industrial GradeView Details
16389-88-1
