D-AMPHETAMINE SULFATE
- CAS NO.:51-63-8
- Empirical Formula: C18H28N2O4S
- Molecular Weight: 368.49
- MDL number: MFCD00069209
- EINECS: 200-111-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:26:57
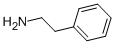
What is D-AMPHETAMINE SULFATE?
Chemical properties
white powder
Originator
Dexedrine Sulfate,SKF,US,1944
The Uses of D-AMPHETAMINE SULFATE
Amfetamine
Manufacturing Process
Two mols, for example, 270 grams, of racemic α-methylphenethylamine base
are reacted with one mol (150 grams) of d-tartaric acid, thereby forming dl-αmethylphenethylamine d-tartrate, a neutral salt. The neutral salt thus
obtained is fully dissolved by the addition of sufficient, say about 1 liter, of
absolute ethanol, and heating to about the boiling point. The solution is then
allowed to cool to room temperature with occasional stirring to effect
crystallization. The crystals are filtered off and will be found to contain a
preponderance of the levo enantiomorph.
The residual solid in the mother liquors is repeatedly and systematically
crystallized, yielding a further fraction of 1-α-methylphenethylamine d-tartrate
which may be purified by recrystallization. d-α-Methylphenethylamine may be
readily recovered from the mother liquors by the addition of tartaric acid
thereto for the formation of acid tartrates and separation of d-αmethylphenethylamine d-bitartrate by crystallization.
The free base of either optical isomer may be obtained by addition to the dtartrate in the case of the levo isomer and the d-bitartrate in the case of the
dextro isomer of alkali in excess, as, for example, by the addition of an
aqueous solution of caustic soda, which will cause the base to separate as an oil which may be recovered and purified by any well-known procedure. The
base is exactly neutralized with sulfuric acid to give the sulfate.
Therapeutic Function
Central stimulant
Biological Activity
CNS stimulant. Targets monoamine transporters to elevate synaptic levels of noradrenalin, dopamine and serotonin.
Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. A human teratogen that causes developmental abnormalities of the central nervous system. Experimental reproductive effects including other teratogenic effects. A habit-forming stimulant. When heated to decomposition it emits very toxic fumes of SO, and NO,. See also other benzidrine compounds and SULFATES.
Storage
Desiccate at RT
Properties of D-AMPHETAMINE SULFATE
| Melting point: | >300° |
| alpha | D20 +21.8° (c = 2) |
| Density | 1.1500 |
| refractive index | 1.6930 (estimate) |
| storage temp. | Desiccate at RT |
| solubility | H2O: soluble |
| form | Powder |
| color | Plates |
| Water Solubility | Soluble to 100 mM in water |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | Benzeneethanamine, .alpha.-methyl-, (.alpha.S)-, sulfate (2:1) (51-63-8) |
Safety information for D-AMPHETAMINE SULFATE
Computed Descriptors for D-AMPHETAMINE SULFATE
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
