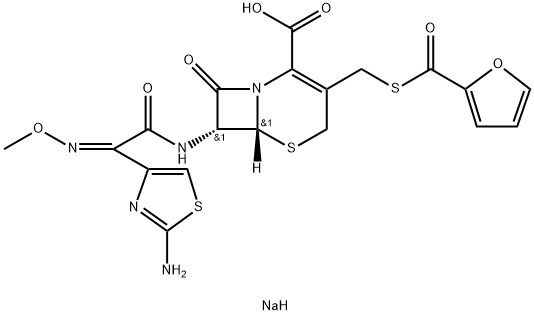
Cefoselis sulfate
- CAS NO.:122841-12-7
- Empirical Formula: C19H24N8O10S3
- Molecular Weight: 620.63
- MDL number: MFCD01940015
- EINECS: 1533716-785-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
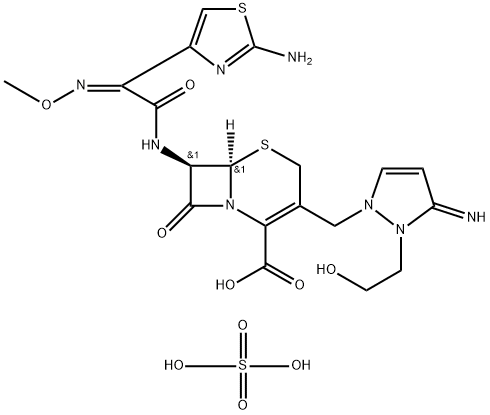
What is Cefoselis sulfate?
Description
Cefoselis is a new fourth-generation cephalosporin and was launched
in Japan as a parenteral antibiotic for a variety of infections including
Staphylococcus aureus (particularly the methicillin-resistant MRSA) and
Pseudornonas aeruginosa. It can be prepared in 3 related ways, all using 2-
pyrazolomethyl-3-cephem-4-carboxylic as the key intermediate. In preclinical
studies, Cefolesis had better MIC50s than Ceftazidime against Streptococcus
pneurnoniae or Staphflococcus aureus (methicillin-sensitive MSSA or resistant
MRSA) and showed significant antibacterial activity against Citrobacter and
Enterobacter.
Cefolesis is well tolerated in clinical studies, being eliminated mainly via
glomerular filtration in humans.
Originator
Fujisawa (Japan)
The Uses of Cefoselis sulfate
antibiotic
Definition
ChEBI: Cefoselis sulfate is an azaheterocycle sulfate salt. It is functionally related to a member of cefoselis.
brand name
Wincef
Properties of Cefoselis sulfate
| storage temp. | 4°C, protect from light, stored under nitrogen |
| solubility | DMSO : 100 mg/mL (161.12 mM; Need ultrasonic)H2O : 14.29 mg/mL (23.02 mM; Need ultrasonic) |
| form | Powder |
| color | White to off-white |
| CAS DataBase Reference | 122841-12-7(CAS DataBase Reference) |
Safety information for Cefoselis sulfate
Computed Descriptors for Cefoselis sulfate
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 Cefoselis sulfate 98% (HPLC) CAS 122841-12-7View Details
Cefoselis sulfate 98% (HPLC) CAS 122841-12-7View Details
122841-12-7 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
