4-Formylphenylboronic acid
Synonym(s):p-Formylbenzeneboronic acid;p-Formylphenylboronic acid;4-(Dihydroxyboryl)benzaldehyde;4-Boronobenzaldehyde;4-Formylbenzeneboronic acid
- CAS NO.:87199-17-5
- Empirical Formula: C7H7BO3
- Molecular Weight: 149.94
- MDL number: MFCD00151823
- EINECS: 438-670-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:12:53
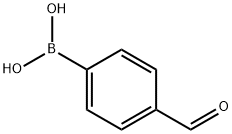
What is 4-Formylphenylboronic acid?
Chemical properties
white to light yellow crystal powder
The Uses of 4-Formylphenylboronic acid
4-Formylphenylboronic acid is a substrate for Suzuki cross-coupling reactions and it can be used as a reagent for:
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Triethylamine-catalyzed three-component Hantzsch condensations.
- Copper-catalyzed nitrations.
- Oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta.
- Palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides.
- Palladium-catalyzed aerobic oxidative cross-coupling reactions.
- The synthesis of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells.
- The synthesis of a novel protein synthesis inhibitor active against Gram-positive bacteria.
- The Suzuki aryl-aryl coupling of the upper rim of hexahomotrioxacalix[3]arene.
- A rhodium-catalyzed cyclization, converting 1,5-enynes to cyclopentenes and spiro-cyclopentenes.
The Uses of 4-Formylphenylboronic acid
suzuki reaction
The Uses of 4-Formylphenylboronic acid
4-Formylbenzeneboronic acid acts as a reagent used for Palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water, copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids, triethylamine-catalyzed three-component Hantzsch condensations, copper-catalyzed nitrations, oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta, palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides, palladium-catalyzed aerobic oxidative cross-coupling reactions. It acts as a reagent used in preparation of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells, a novel protein synthesis inhibitor active against Gram-positive bacteria.
What are the applications of Application
4-Formylphenylboronic acid is used for various catalytic reactions
Flammability and Explosibility
Not classified
Synthesis
Potassium phenyltrifluoroborate (184 mg, 1.00 mmol) was added to a solution of iron trichloride (185 mg, 1.10 mmol) in 3 mL of 1:1 THF/water. The mixture was stirred at room temperature for 30 min. The reaction mixture was then passed through a short column containing neutral absorption alumina. The alumina was then washed with a mixture of ethyl acetate/hexanes (2:1) to obtain 4-Formylphenylboronic acid (105 mg, 86%). [The boronic acid products can also be isolated by simple extraction techniques.]
Properties of 4-Formylphenylboronic acid
| Melting point: | 237-242 °C (lit.) |
| Boiling point: | 347.6±44.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | <10g/l |
| form | Liquid |
| pka | 7.34±0.10(Predicted) |
| color | Clear colorless to yellow-orange |
| PH | 5.5 (1g/l, H2O, 20℃) |
| Water Solubility | Slightly Soluble in water. |
| Sensitive | Air Sensitive |
| BRN | 3030770 |
| CAS DataBase Reference | 87199-17-5(CAS DataBase Reference) |
| EPA Substance Registry System | Boronic acid, (4-formylphenyl)- (87199-17-5) |
Safety information for 4-Formylphenylboronic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for 4-Formylphenylboronic acid
| InChIKey | VXWBQOJISHAKKM-UHFFFAOYSA-N |
4-Formylphenylboronic acid manufacturer
JSK Chemicals
SAKEM LLP
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran


You may like
-
 4-FORMYL PHENYL BORONIC ACID 87199-17-5 98%View Details
4-FORMYL PHENYL BORONIC ACID 87199-17-5 98%View Details
87199-17-5 -
 87199-17-5 99%View Details
87199-17-5 99%View Details
87199-17-5 -
 4-(Formylphenyl) boronic acid 87199-17-5 98%View Details
4-(Formylphenyl) boronic acid 87199-17-5 98%View Details
87199-17-5 -
 4-Formylphenylboronic Acid (contains varying amounts of Anhydride) CAS 87199-17-5View Details
4-Formylphenylboronic Acid (contains varying amounts of Anhydride) CAS 87199-17-5View Details
87199-17-5 -
 4-Formylphenylboronic Acid extrapure CAS 87199-17-5View Details
4-Formylphenylboronic Acid extrapure CAS 87199-17-5View Details
87199-17-5 -
 4-Formylphenylboronic acid, 97% CAS 87199-17-5View Details
4-Formylphenylboronic acid, 97% CAS 87199-17-5View Details
87199-17-5 -
 4-Formylphenylboronic acid CAS 87199-17-5View Details
4-Formylphenylboronic acid CAS 87199-17-5View Details
87199-17-5 -
 87199-17-5 4-Formyl Phenyl Boronic Acid 98%View Details
87199-17-5 4-Formyl Phenyl Boronic Acid 98%View Details
87199-17-5
