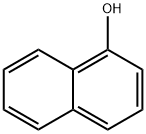
2-Nitro-1-naphthol
- CAS NO.:607-24-9
- Empirical Formula: C10H7NO3
- Molecular Weight: 189.17
- MDL number: MFCD00003956
- EINECS: 210-131-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:20:01
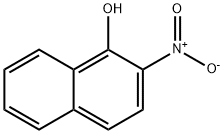
What is 2-Nitro-1-naphthol?
Description
2-Nitro-1-naphthol is an important organic compound with anti-inflammatory properties used as an analytical method for measuring albumin concentration and as an enzyme-labelled substrate in electrochemical immunoassays. The substance reacts with albumin to form a coloured complex which can be detected spectrophotometrically. It is also used as a model system for the study of inflammatory diseases and inhibits p38 kinase activity. In addition, a derivative of 2-Nitro-1-naphthol (4-nitro-1-naphthol) can be used to prepare chromogenic substrates for neutral or slightly acidic hydrolases.
Chemical properties
yellow-green to ochre-brown powder and/or chunks
The Uses of 2-Nitro-1-naphthol
2-Nitro-1-naphthol was employed as substrate for use in an amperometric 3-electrode system for the determination of alkaline phosphatase, the enzyme label most commonly used in electrochemical immunoassays. It was also used in the preparation of:
- di-(2-nitro-1-naphthyl) hydrogen phosphate
- 1-(2′-methyl-3′-indenyl)-2-naphthylamine and -2-naphthol (axially chiral ligands)
What are the applications of Application
2-Nitro-1-naphthol is commonly used as an intermediate in organic synthesis. It is a derivative of 1-nitro-2-naphthol and 4-nitro-1-naphthol. 1-Nitro-2-naphthol has been found to be a potent inhibitor of enzyme-dependent activation of acetyl-CoA, while 4-nitro-1-naphthol facilitates the preparation of chromogenic substrates for neutral or slightly acidic hydrolases[1-2].
Purification Methods
Crystallise the naphthol (repeatedly) from EtOH. [Beilstein 6 H 615, 6 III 2938, 6 IV 4236.]
References
[1] JIZHENG DANG . Chromogenic substrate from 4-nitro-1-naphthol for hydrolytic enzyme of neutral or slightly acidic optimum pH: 4-Nitro-1-naphthyl-β-d-galactopyranoside as an example[J]. Bioorganic & Medicinal Chemistry Letters, 2013. DOI:10.1016/j.bmcl.2012.11.120.
[2] ATSUKO SHINOHARA. Inhibition of acetyl-coenzyme A dependent activation of N-hydroxyarylamines by phenolic compounds, pentachlorophenol and 1-nitro-2-naphthol[J]. Chemico-Biological Interactions, 1986. DOI:10.1016/0009-2797(86)90058-X.
Properties of 2-Nitro-1-naphthol
| Melting point: | 123-125 °C (lit.) |
| Boiling point: | 324.41°C (rough estimate) |
| Density | 1.3175 (rough estimate) |
| refractive index | 1.5400 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| pka | 6.13±0.50(Predicted) |
| InChI | InChI=1S/C10H7NO3/c12-10-8-4-2-1-3-7(8)5-6-9(10)11(13)14/h1-6,12H |
| CAS DataBase Reference | 607-24-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Nitro-1-naphthol(607-24-9) |
Safety information for 2-Nitro-1-naphthol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Nitro-1-naphthol
| InChIKey | MUCCHGOWMZTLHK-UHFFFAOYSA-N |
| SMILES | C1(O)=C2C(C=CC=C2)=CC=C1[N+]([O-])=O |
New Products
Cyclopentane-1,2-dione 2,6-Dibromoaniline (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 1-AMino-cyclobutaneMethanol HCl TETRABUTYLAMMONIUM CYANIDE 3-Amino-3-(3-fluorophenyl)propanoic acid 3-Amino-3-(4-methylphenyl)propionic acid 3-AMINO-3-(3-CHLORO-PHENYL)-PROPIONIC ACID Cefuroxime EP Impurity-A N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 4 - fluoro- 2 - methoxy- 5 - nitroaniline 1-(2-amino-5-hydroxy phenyl)propan-1-one tert-butyl 4-(6-((7-cyclo pentyl -6-(dimethyl carbamoyl) -7H-pyrrolo [2,3-d]pyrimidin-2-yl) amino)pyridin-3-yl) piperazine-1-carboxylate 2-amino-5-nitrobenzonitrile (4-Fluoro-2-methoxy-5-nitro- phenyl)-[4- (1-methyl-1H- indol-3- yl)-pyrimidin-2-yl]- amine (3R,4R)-1-benzyl-N,4-dimethylpiperidin-3- amine 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanideRelated products of tetrahydrofuran
You may like
-
 2-Nitro-1-naphthol CAS 607-24-9View Details
2-Nitro-1-naphthol CAS 607-24-9View Details
607-24-9 -
 10442-39-4 TETRABUTYLAMMONIUM CYANIDE 98+View Details
10442-39-4 TETRABUTYLAMMONIUM CYANIDE 98+View Details
10442-39-4 -
 3-Amino-3-(3-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(3-fluorophenyl)propanoic acid 98+View Details
117391-51-2 -
 3-Amino-3-(4-methylphenyl)propionic acid 68208-18-4 98+View Details
3-Amino-3-(4-methylphenyl)propionic acid 68208-18-4 98+View Details
68208-18-4 -
 10442-39-4 TETRABUTYLAMMONIUM CYANIDE 98+View Details
10442-39-4 TETRABUTYLAMMONIUM CYANIDE 98+View Details
10442-39-4 -
 68208-21-9 98+View Details
68208-21-9 98+View Details
68208-21-9 -
 3-Amino-3-(3-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(3-fluorophenyl)propanoic acid 98+View Details
117391-51-2 -
 3-Amino-3-(4-methylphenyl)propionic acid 68208-18-4 98+View Details
3-Amino-3-(4-methylphenyl)propionic acid 68208-18-4 98+View Details
68208-18-4
