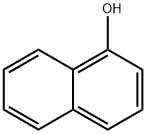
2-Nitro-1-naphthol
- CAS NO.:607-24-9
- Empirical Formula: C10H7NO3
- Molecular Weight: 189.17
- MDL number: MFCD00003956
- EINECS: 210-131-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:20:01
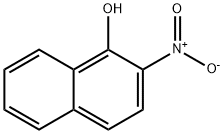
What is 2-Nitro-1-naphthol?
Description
2-Nitro-1-naphthol is an important organic compound with anti-inflammatory properties used as an analytical method for measuring albumin concentration and as an enzyme-labelled substrate in electrochemical immunoassays. The substance reacts with albumin to form a coloured complex which can be detected spectrophotometrically. It is also used as a model system for the study of inflammatory diseases and inhibits p38 kinase activity. In addition, a derivative of 2-Nitro-1-naphthol (4-nitro-1-naphthol) can be used to prepare chromogenic substrates for neutral or slightly acidic hydrolases.
Chemical properties
yellow-green to ochre-brown powder and/or chunks
The Uses of 2-Nitro-1-naphthol
2-Nitro-1-naphthol was employed as substrate for use in an amperometric 3-electrode system for the determination of alkaline phosphatase, the enzyme label most commonly used in electrochemical immunoassays. It was also used in the preparation of:
- di-(2-nitro-1-naphthyl) hydrogen phosphate
- 1-(2′-methyl-3′-indenyl)-2-naphthylamine and -2-naphthol (axially chiral ligands)
What are the applications of Application
2-Nitro-1-naphthol is commonly used as an intermediate in organic synthesis. It is a derivative of 1-nitro-2-naphthol and 4-nitro-1-naphthol. 1-Nitro-2-naphthol has been found to be a potent inhibitor of enzyme-dependent activation of acetyl-CoA, while 4-nitro-1-naphthol facilitates the preparation of chromogenic substrates for neutral or slightly acidic hydrolases[1-2].
Purification Methods
Crystallise the naphthol (repeatedly) from EtOH. [Beilstein 6 H 615, 6 III 2938, 6 IV 4236.]
References
[1] JIZHENG DANG . Chromogenic substrate from 4-nitro-1-naphthol for hydrolytic enzyme of neutral or slightly acidic optimum pH: 4-Nitro-1-naphthyl-β-d-galactopyranoside as an example[J]. Bioorganic & Medicinal Chemistry Letters, 2013. DOI:10.1016/j.bmcl.2012.11.120.
[2] ATSUKO SHINOHARA. Inhibition of acetyl-coenzyme A dependent activation of N-hydroxyarylamines by phenolic compounds, pentachlorophenol and 1-nitro-2-naphthol[J]. Chemico-Biological Interactions, 1986. DOI:10.1016/0009-2797(86)90058-X.
Properties of 2-Nitro-1-naphthol
| Melting point: | 123-125 °C (lit.) |
| Boiling point: | 324.41°C (rough estimate) |
| Density | 1.3175 (rough estimate) |
| refractive index | 1.5400 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| pka | 6.13±0.50(Predicted) |
| InChI | InChI=1S/C10H7NO3/c12-10-8-4-2-1-3-7(8)5-6-9(10)11(13)14/h1-6,12H |
| CAS DataBase Reference | 607-24-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Nitro-1-naphthol(607-24-9) |
Safety information for 2-Nitro-1-naphthol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Nitro-1-naphthol
| InChIKey | MUCCHGOWMZTLHK-UHFFFAOYSA-N |
| SMILES | C1(O)=C2C(C=CC=C2)=CC=C1[N+]([O-])=O |
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 2-Nitro-1-naphthol CAS 607-24-9View Details
2-Nitro-1-naphthol CAS 607-24-9View Details
607-24-9 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
