2-Naphthol
Synonym(s):β-Naphthol;2-Hydroxy naphthaline;2-Hydroxynaphthalene;2-Naphthol
- CAS NO.:135-19-3
- Empirical Formula: C10H8O
- Molecular Weight: 144.17
- MDL number: MFCD00004067
- EINECS: 205-182-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:27:03
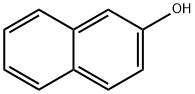
What is 2-Naphthol?
Chemical properties
2-Naphthol[135-19-3] is a white, crystalline solid. Slight phenolic odor. Darkens in air and on exposure to light. Unlike 1-naphthol it is nonvolatile in steam. 2-Naphthol is reduced with sodium or with hydrogen in the presence of a catalyst to give mainly 1,2,3,4-tetrahydro-2-naphthol (unlike 1- naphthol which gives the arylphenol under the same conditions). Oxidation with ferric chloride forms 2,20 -dihydroxy-1,10 -binaphthyl, with any blue coloration indicating the presence of 1- naphthol. Air oxidation at 300℃ with a vanadium pentoxide catalyst also yields 1,10 -bi-2-naphthol, which dehydrates at higher temperature to the oxide and finally decomposes to give benzoic acid. Reaction with phosphorus pentachloride at 150℃ gives 2-chloronaphthalene or at lower temperature, tri-2-naphthyl phosphate (mp 111℃), which can be isolated after treatment of the reaction mixture with alkali.
Physical properties
White glossy flakes or white powder. Insoluble in water, soluble in ethanol, ether, chloroform, glycerol and alkali solution.
The Uses of 2-Naphthol
2-Naphthol is used in the manufacture of dyes, perfumes, and medicinal organics, and in the production of antioxidants for synthetic rubber.
Definition
ChEBI: 2-naphthol is a naphthol carrying a hydroxy group at position 2. It has a role as an antinematodal drug, a genotoxin, a human xenobiotic metabolite, a mouse metabolite, a human urinary metabolite and a radical scavenger.
Preparation
2-Naphthol is produced by
caustic fusion of naphthalene-2-sulfonic acid. Typically, the sodium salt of the
sulfonic acid is added gradually to 50 % sodium
hydroxide liquor at 300℃; the melt is then
heated further at 320℃ in a gas-fired iron vessel
with vigorous agitation. After completion of the
reaction, the melt is run into excess water, possibly including filtrate from the previous batch at a
proven tolerable level, and the naphtholate solution is neutralized to pH 8 with dilute sulfuric
acid. If the temperature is maintained at >100℃ during neutralization, the crude product comes
out of solution as an oil, which is separated,
washed with hot water, and distilled under vacuum to give pure 2-naphthol. The molten material
is processed through a flaker to give the final
product for packaging. The fusion yield is about
80 % of the theoretical value, resulting in an
overall yield of 70 % based on naphthalene.
Typical specifications for 2-naphthol are clear
solution in dilute caustic soda, mp 120.5℃,
and 1-naphthol content<0.3 %.
The newer method of manufacture is economically and environmentally favored in the United States, because despite requiring three stages, it is more amenable to
continuous operation with recycle streams. The
alkylation and isomerization are carried out up to
240℃ with a phosphoric acid catalyst.
Final catalytic oxidation at 90 – 110℃ gives
the hydroperoxide, which is cleaved with dilute
sulfuric acid to give 2-naphthol in high overall
yield in spite of modest oxidation conversion.
Reactions
2-Naphthol is naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols.2-Naphthol converts to 2-naphthalenethiol by reaction with dimethylthiocarbamoyl chloride via the Newman–Kwart rearrangement.
Synthesis Reference(s)
Journal of the American Chemical Society, 72, p. 4884, 1950 DOI: 10.1021/ja01167a009
Synthesis, p. 437, 1985 DOI: 10.1055/s-1985-31235
General Description
2-Naphthol is a hydroxyarene molecule, which when electronically excited forms strong acid. Excited it dissociates only in water. It has a slight phenolic odor. It is incompatible with strong oxidizing agents, strong bases, acid chlorides and acid anhydrides. It is one of the most commonly used fluorescence dye.
Health Hazard
Although the toxicity of 2-naphthol is of low order in test animals, ingestion of large amounts may result in nausea, vomiting, diarrhea, abdominal pain, convulsions, and hemolytic anemia. Death may result from respiratory failure. The oral LD50 value in rats is in the range 2000 mg/kg. 2-Naphthol is slightly more toxic than 1-naphthol [9015-3], the oral LD50 value of which is in the range 2500 mg/kg.
Skin contact can produce peeling of the skin and pigmentation.
Fire Hazard
Noncombustible solid.
Safety Profile
2-Naphthol is poison by ingestion, inhalation, and subcutaneous routes. Mutation data reported. A skin and eye irritant. Combustible when exposed to heat or flame. To fight fire, use CO2, dry chemical. Incompatible with antipyrine, camphor, phenol, ferric salts, menthol, potassium permanganate and other oxidzing materials, urethane.
Potential Exposure
A potential danger to those involved in rubber antioxidant production, synthesis of dyes; leather processing; fungicides, pharmaceuticals, and perfumes. Used as an antioxidant for fats, oils; as an antiseptic; in insecticides.
2-Naphthol Application
2-Naphthol is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is one of naphthalene homologues of phenol, but more reactive. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Crystallise 2-naphthol from aqueous 25% EtOH (charcoal), H2O, *benzene, toluene or CCl4. Alternatively, extract it repeatedly with small amounts of EtOH, followed by dissolution in a minimum volume of EtOH and precipitation with distilled water, then drying over P2O5 under vacuum. It has also been dissolved in aqueous NaOH and precipitated by adding acid (repeat several times), then precipitated from *benzene by addition of heptane. Final purification can be by zone melting or sublimation in vacuo. The 4-nitrobenzoate has m 104o (from EtOH). [Bardez et al. J Phys Chem 89 5031 1985, Kikuchi et al. J Phys Chem 91 574 1987, Beilstein 6 IV 4253.]
Properties and Applications
2-Naphthol is a white crystalline, with phenol smell. Industrial goods for hoar chip or powder. Melting point 121 ~ 123 ℃, 285 ℃ ~ 286 boiling point, flash point 161 ℃. This product soluble in water, ethanol, ethyl ether, chloroform, glycerin and alkali solution, can the sublimation, with steam evaporate out; Long storage or light color in turn dark, can happen oxidation, reduction, nitrification and nitrosation reaction, and products, light and heat, halogenating role, etc.
Incompatibilities
Dust or powder may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, iron salts; 2,3-dimethyl-1- phenyl-3-pyrazolin-5-one (antipyrine); camphor, phenol, menthol, urethane.
Waste Disposal
Mix with flammable solvent and atomize into an incinerator.
Properties of 2-Naphthol
| Melting point: | 120-122 °C(lit.) |
| Boiling point: | 285-286 °C(lit.) |
| Density | 1,28 g/cm3 |
| vapor density | 4.97 (vs air) |
| vapor pressure | 10 mm Hg ( 145.5 °C) |
| refractive index | 1.5762 (estimate) |
| Flash point: | 153 °C |
| storage temp. | Store below +30°C. |
| solubility | methanol: soluble1g/10 mL, clear, colorless to light yellow |
| form | Powder, Crystals or Granules |
| pka | 9.51(at 25℃) |
| color | White |
| PH Range | Non& uorescence (8.5) to blue & uorescence (9.5) |
| Odor | faint phenol-like odor |
| Water Solubility | 1 g/L (20 ºC) |
| λmax | 226nm, 265nm, 275nm, 286nm, 320nm, 331nm |
| Merck | 14,6384 |
| BRN | 742134 |
| Stability: | Stable. Combustible. Dust may form explosive mixture with air. Incompatible with strong oxidizing agents, phenol. |
| Major Application | Display device, semiconductors, photoimaging materials, inks, toner, chalk, security paper, molding materials, tin plating method, rubber, adhesive, leather, detergent, hair dyes, antimitotic drug, anticancer agent, antiinflammatory agent, treatment of acne vulgaris (pimples) and other dermal ailments (rashes, scratches, blemishes, hair loss), disorders |
| CAS DataBase Reference | 135-19-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Naphthalenol(135-19-3) |
| EPA Substance Registry System | 2-Naphthalenol (135-19-3) |
Safety information for 2-Naphthol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Naphthol
| InChIKey | JWAZRIHNYRIHIV-UHFFFAOYSA-N |
2-Naphthol manufacturer
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
![(2R,3R)-2-[(1R)-1-[3,5-Bis(trifluoroMethyl)phenyl]ethoxy]-3-(4-fluorophenyl)Morpholine](https://img.chemicalbook.in/CAS/20150408/GIF/380499-06-9.gif)
![2-(2-(4-(Dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl)ethoxy)ethyl Acetate](https://img.chemicalbook.in/CAS/20150408/GIF/844639-07-2.gif)
![2-(2-(4-(Dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl)ethoxy)ethanol](https://img.chemicalbook.in/CAS/20150408/GIF/848814-27-7.gif)


You may like
-
 BETA NAPTHOL 99%View Details
BETA NAPTHOL 99%View Details -
 2-Naphthol (SQ) CAS 135-19-3View Details
2-Naphthol (SQ) CAS 135-19-3View Details
135-19-3 -
 ß-Naphthol extrapure AR CAS 135-19-3View Details
ß-Naphthol extrapure AR CAS 135-19-3View Details
135-19-3 -
 2-Naphthol CAS 135-19-3View Details
2-Naphthol CAS 135-19-3View Details
135-19-3 -
 Naphthalen-2-ol 98% CAS 135-19-3View Details
Naphthalen-2-ol 98% CAS 135-19-3View Details
135-19-3 -
 2-Naphthol, Packaging Type: DrumView Details
2-Naphthol, Packaging Type: DrumView Details
135-19-3 -
 2 NaphtholView Details
2 NaphtholView Details
135-19-3 -
 b-NAPHTHOL 99% (For Synthesis) (500 gm), Powder, BottleView Details
b-NAPHTHOL 99% (For Synthesis) (500 gm), Powder, BottleView Details
135-19-3
