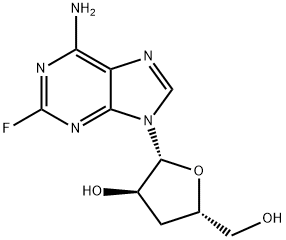
2-Fluoroadenosine
- CAS NO.:146-78-1
- Empirical Formula: C10H12FN5O4
- Molecular Weight: 285.23
- MDL number: MFCD00866394
- EINECS: 633-923-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:23:27
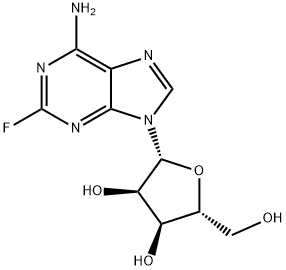
What is 2-Fluoroadenosine?
Description
2-Fluoroadenosine (F-Ado) was developed at Southern Research Institute in 1957 as a potential anticancer drug. 2-Fluoroadenosine is not deaminated by adenosine deaminase but metabolized to triphosphate as shown in vitro. The drug was also shown to be a potent inhibitor of lymphocyte-mediated cytolysis.
The Uses of 2-Fluoroadenosine
2-Fluoroadenosine is a fluorinated analog of Adenoside nucleotide. It is used as an intermediate for the drug fludarabine. Fludarabine is a purine analogue and antineoplastic agent. It is a chemotherapy medication used in the treatment of leukemia and lymphoma.
Definition
ChEBI: 2-fluoroadenosine is a member of adenosines and an organofluorine compound.
Biological Functions
2-Fluoroadenosine (F-Ado) was synthesized by Montgomery and his co-workers. This compound has been found to suppress the growth of H.Ep. No. 2 cells growing in culture. Bennett and Smithers have shown that in the same cells, the synthesis of 5-phosphoribosylamine was markedly inhibited by both 2- fluoroadenine and F-Ado. The compound was found to inhibit the incorporation of variously labeled precursors into ribonucleic acid in whole Ehrlich ascites cells. Upon incubation with ascites cells, the nucleoside was readily converted to the 5’-mono-, 5’-di-, and 5’-triphosphates.In addition, striking potentiation of the inhibition of human platelet aggregation by combinations of forskolin with PGE 1 or F-Ado [1-2].
Synthesis
A novel method for the introduction of fluorine in the purine 2-position is described and employed in the synthesis of a potential antimycobacterial compound. Also 2-fluoroadenosine has been synthesized for the first time from adenosine with perbenzoylated 2-nitroadenosine as a key intermediate. Mild reaction conditions are employed and few synthetic steps are required. The novel synthesis of fluoroadenosine can be regarded as a formal synthesis of the antileukemic drug fludarabine phosphate.
[1] MORTEN BR?NDVANG L. G. A Novel Method for the Introduction of Fluorine into the Purine 2-Position: Synthesis of 2-Fluoroadenosine and a Formal Synthesis of the Antileukemic Drug Fludarabine[J]. Synthesis-Stuttgart, 2006, 87 1: 2993-2995. DOI:10.1055/S-2006-942544.
References
[1] H T Shigeura. “Metabolism of 2-fluoroadenosine by Ehrlich ascites cells.” Archives of biochemistry and biophysics 111 3 (1965): 713–9.
[2] Kailash C. Agarwal, Robert E. Parks Jr. “Synergistic inhibition of platelet aggregation by forskolin plus PGE1 or 2-fluoroadenosine: Effects 2′,5′-dideoxyadenosine and 5′-methylthioadenosine.” Biochemical pharmacology 31 22 (1982): Pages 3713-3716.
Properties of 2-Fluoroadenosine
| Melting point: | 240 °C (D)(lit.) |
| Boiling point: | 747.3±70.0 °C(Predicted) |
| Density | 2.17±0.1 g/cm3(Predicted) |
| refractive index | -72 ° (C=0.1, EtOH) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly, Sonicated), Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 13.05±0.70(Predicted) |
| color | Off-White |
| InChI | InChI=1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1 |
| CAS DataBase Reference | 146-78-1(CAS DataBase Reference) |
Safety information for 2-Fluoroadenosine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Fluoroadenosine
| InChIKey | HBUBKKRHXORPQB-UUOKFMHZSA-N |
| SMILES | OC[C@H]1O[C@@H](N2C3C(=C(N=C(F)N=3)N)N=C2)[C@H](O)[C@@H]1O |
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 2-Fluoroadenosine CAS 146-78-1View Details
2-Fluoroadenosine CAS 146-78-1View Details
146-78-1 -
 2-Fluoroadenosine CAS 146-78-1View Details
2-Fluoroadenosine CAS 146-78-1View Details
146-78-1 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
