1-Naphthylamine-4,6,8-trisulfonic acid
- CAS NO.:17894-99-4
- Empirical Formula: C10H9NO9S3
- Molecular Weight: 383.37
- MDL number: MFCD00035848
- EINECS: 241-837-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-06-20 14:58:42
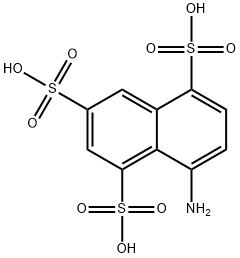
What is 1-Naphthylamine-4,6,8-trisulfonic acid?
Chemical properties
1-Aminonaphthalene-4,6,8-trisulfonic acid [17894-99-4]. (8-aminonaphthalene1,3,5-trisulfonic acid), B acid, C10H9NO9S3, Mr 383.36, is reduced with zinc and alkali to 1- aminonaphthalene-4,6-disulfonic acid. Heating with aqueous sulfuric acid causes desulfonation to form 1-aminonaphthalene-6,8-disulfonic acid. Sulfonation (25 % oleum, 90℃) gives the sultam of 1-aminonaphthalene-2,4,6,8-tetrasulfonic acid.
The Uses of 1-Naphthylamine-4,6,8-trisulfonic acid
Alkali fusion of B acid yields K acid. A special outlet for purified B acid is in the synthesis of the drug suramin sodium (Bayer 205) . 1-Aminonaphthalene-4,6,8-trisulfonic acid is acylated with 2-nitrotoluyl chloride, and this product is reduced and acylated with mnitrobenzoyl chloride. This intermediate is reduced and finally phosgenated to give suramin sodium, which has been used to treat trypanosomiasis and onchocerciasis.
Production Methods
Naphthalene is sulfonated under conditions that maximize formation of the 1,3,5-trisulfonic acid, followed by nitration with H2SO4– HNO3 in situ at 35℃. After quenching, neutralization with calcium carbonate, and reduction of the nitro-derivative with iron under acid conditions, the byproduct 1- aminonaphthalene-3,6,8-trisulfonic acid (18 % yield) is selectively precipitated by acidification. The desired isomer is then precipitated by further acidification and salting of the filtrates, and finally purified by reprecipitation. Overall yield is 25 – 30 %.
Properties of 1-Naphthylamine-4,6,8-trisulfonic acid
| Density | 1.974±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| pka | -1.75±0.40(Predicted) |
Safety information for 1-Naphthylamine-4,6,8-trisulfonic acid
Computed Descriptors for 1-Naphthylamine-4,6,8-trisulfonic acid
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
