Trifluoromethanesulfonic anhydride
Synonym(s):Perfluoromethanesulfonic anhydride solution;Tf2O;Triflic anhydride;Triflic anhydride solution;Trifluoromethanesulfonic anhydride
- CAS NO.:358-23-6
- Empirical Formula: C2F6O5S2
- Molecular Weight: 282.14
- MDL number: MFCD00000408
- EINECS: 206-616-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:22:24
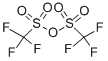
What is Trifluoromethanesulfonic anhydride?
Description
Trifluoromethanesulfonic anhydride, also known as triflic anhydride, has proven to be an extraordinary reagent for a broad range of transformations. As a commercially and readily available reagent, It has been widely used in synthetic chemistry due to its high electrophilicity.
Chemical properties
clear colorless to light brown liquid
The Uses of Trifluoromethanesulfonic anhydride
Trifluoromethanesulfonic Anhydride is a strong electrophile used in chemical synthesis for introducing the triflyl group. Trifluoromethanesulfonic anhydride is used to convert phenols and imine into triflic ester and NTf group. It serves as a reagent in the preparation of alkyl and vinyl triflates, and for the stereoselective synthesis of mannosazide methyl uronate donors. It acts as a catalyst for glycosylation with anomeric hydroxy sugars to prepare polysaccharides.
Definition
ChEBI: Triflic anhydride is an organosulfonic anhydride. It is functionally related to a triflic acid.
Hazard
May be corrosive to metals. Harmful if swallowed. Causes severe skin burns and eye damage.
Storage
Store in a cool, dry, wellventilated area. Moisture sensitive.
Purification Methods
It can be freshly prepared from the anhydrous acid (11.5g) and P2O5 (11.5g, or half this weight) by setting aside at room temperature for 1hour, distilling off volatile products then distil it through a short Vigreux column. It is readily hydrolysed by H2O and decomposes appreciably after a few days to liberate SO2 and produce a viscous liquid. Store it dry at low temperatures. [Burdon et al. J Chem Soc 2574 1957, Beard et al. J Org Chem 38 373 1973, Beilstein 3 IV 35.]
References
[1] Haoqi Zhang. “Trifluoromethanesulfonic Anhydride in Amide Activation: A Powerful Tool to Forge Heterocyclic Cores.” TCIMAIL (2021).
[2] Dr. Qixue Qin, Prof. Dr. Ning Jiao, Zengrui Cheng. “Recent Applications of Trifluoromethanesulfonic Anhydride in Organic Synthesis.” Angewandte Chemie 135 10 (2022).
Properties of Trifluoromethanesulfonic anhydride
| Melting point: | -80°C |
| Boiling point: | 81-83 °C (lit.) |
| Density | 1.677 g/mL at 25 °C (lit.) |
| vapor density | 5.2 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| refractive index | n |
| RTECS | PB2772000 |
| Flash point: | 81-83°C |
| storage temp. | Store below +30°C. |
| solubility | Miscible with dichloromethane. Immiscible with hydrocarbons. |
| form | Liquid |
| color | Clear colorless to light brown |
| Specific Gravity | 1.677 |
| Water Solubility | reacts violently with water |
| Sensitive | Moisture Sensitive |
| BRN | 1813600 |
| Stability: | Hygroscopic, Moisture Sensitive |
| CAS DataBase Reference | 358-23-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Trifluoromethanesulfonic anhydride(358-23-6) |
| EPA Substance Registry System | Methanesulfonic acid, trifluoro-, anhydride (358-23-6) |
Safety information for Trifluoromethanesulfonic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trifluoromethanesulfonic anhydride
| InChIKey | WJKHJLXJJJATHN-UHFFFAOYSA-N |
Trifluoromethanesulfonic anhydride manufacturer
JSK Chemicals
Chimerical
GLR Innovations
ASM Organics
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Trifluoromethanesulfonic anhydride supplierView Details
Trifluoromethanesulfonic anhydride supplierView Details
358-23-6 -
 Trifluoromethane sulfonic anhydride 98%View Details
Trifluoromethane sulfonic anhydride 98%View Details -
 Trifluoromethanesulfonic anhydride, 99% CAS 358-23-6View Details
Trifluoromethanesulfonic anhydride, 99% CAS 358-23-6View Details
358-23-6 -
 Trifluoromethanesulfonic Anhydride CAS 358-23-6View Details
Trifluoromethanesulfonic Anhydride CAS 358-23-6View Details
358-23-6 -
 Trifluoromethanesulfonic anhydride 98% CAS 358-23-6View Details
Trifluoromethanesulfonic anhydride 98% CAS 358-23-6View Details
358-23-6 -
 Trifluoromethanesulfonic anhydride CAS 358-23-6View Details
Trifluoromethanesulfonic anhydride CAS 358-23-6View Details
358-23-6 -
 Trifluoromethanesulfonic anhydride solution CAS 358-23-6View Details
Trifluoromethanesulfonic anhydride solution CAS 358-23-6View Details
358-23-6 -
 GLR Trifluoromethanesulfonic Anhydride,Cas No-358-23-6, Grade: Lab Grade, Packaging Size: Gm,KGView Details
GLR Trifluoromethanesulfonic Anhydride,Cas No-358-23-6, Grade: Lab Grade, Packaging Size: Gm,KGView Details
358-23-6
