1-Deoxynojirimycin
Synonym(s):1,5-Dideoxy-1,5-imino-D -sorbitol;DNM;DNM1
- CAS NO.:19130-96-2
- Empirical Formula: C6H13NO4
- Molecular Weight: 163.17
- MDL number: MFCD00063474
- EINECS: 606-239-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-26 13:56:28
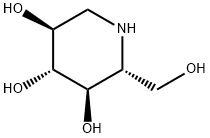
What is 1-Deoxynojirimycin?
Description
Deoxynojirimycin (19130-96-2) inhibits α-glucosidase I and II.1,2 Inhibits human immunodeficiency virus envelope glycoprotein-mediated membrane fusion at the CXCR4 binding step.3 May be used to produce an affinity ligand for purifying glucosidase 1.4 Deoxynojirimycin was used to inhibit ER glucosidases I and II allowing for the discovery of a second mechanism for deglucosylation of N-linked oligosaccharides in PhaR1.7, a mouse lymphoma cell line.5
Chemical properties
White Crystalline Solid
The Uses of 1-Deoxynojirimycin
An alpha-glucosidase inhibitor. Interferes with normal processing of N-linked glycoproteins.(+)-1-Deoxynojirimycin acts as an inhibitor of alfa-glucosidase I and II and maltase-glucoamylase. It also inhibits mammalian glucosidase, intestinal and lysosmal, beta-glucosidase from sweet almonds, pancreatic alfa-amylase and amyloglucosidase. Further, it serves as a enzyme enhancer for the treatment of Fabry and Pompe disease.
The Uses of 1-Deoxynojirimycin
Deoxynojirimycin inhibits mammalian glucosidase 1. As well, it inhibits intestinal and lysosmal alpha-glucosidases, beta-glucosidase from sweet almonds, pancreatic alpha-amylase and amyloglucosidase.
The Uses of 1-Deoxynojirimycin
An inhibitor of α-glucosidase I and II
What are the applications of Application
Deoxynojirimycin is an inhibitor of α-glucosidase I and II
Definition
ChEBI: An optically active form of 2-(hydroxymethyl)piperidine-3,4,5-triol having 2R,3R,4R,5S-configuration.
Biological Activity
Inhibitor of glucosidase I (K i = 2.1 mM) and II (K i = 7 mM).
Storage
+4°C
References
1) Fuhrmann et al. (1985), Inhibitors of oligosaccharide processing; Biochim. Biophys, Acta, 825 95 2) Hughs and Rudge (1994), Deoxynojirimycin: synthesis and biological activity; Nat. Prod. Rep., 11 135 3) Papandreou et al. (2002), The alpha-glucosidase inhibitor 1-deoxynojirimycin blocks human immunodeficiency virus envelope glycoprotein-mediated membrane fusion at the CXCR4 binding step; Mol. Pharmacol., 61 186 4) Hettkamp et al. (1984), Purification by affinity chromatography of glucosidase I, an endoplasmic reticulum hydrolase involved in the processing of asparagine-linked oligosaccharides; Eur. J. Biochem., 142 85 5) Suh et al. (1992), Identification of a novel mechanism for the removal of glucose residues from high mannose-type oligosaccharides; J. Biol. Chem., 267 21671
Properties of 1-Deoxynojirimycin
| Melting point: | 195-196°C |
| Boiling point: | 361.1±42.0 °C(Predicted) |
| Density | 1.456±0.06 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| RTECS | TN4350300 |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in Water up to 25 mg/ml). |
| form | Powder |
| pka | 13.77±0.70(Predicted) |
| color | White |
| Water Solubility | Soluble in water, dimethyl sulfoxide and methanol. |
| BRN | 3588039 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 19130-96-2(CAS DataBase Reference) |
Safety information for 1-Deoxynojirimycin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 1-Deoxynojirimycin
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 (+)-1-Deoxynojirimycin CAS 19130-96-2View Details
(+)-1-Deoxynojirimycin CAS 19130-96-2View Details
19130-96-2 -
 1-Deoxynojirimycin, ≥95% CAS 19130-96-2View Details
1-Deoxynojirimycin, ≥95% CAS 19130-96-2View Details
19130-96-2 -
 1-Deoxynojirimycin CAS 19130-96-2View Details
1-Deoxynojirimycin CAS 19130-96-2View Details
19130-96-2 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
