CHEMICAL AND PHYSICAL PROPERTIES
| LogP | -2.05 |
|---|---|
| Ionization Efficiency | Positive |
| Collision Cross Section | 212.3 Ų [M-H]- 223.51 Ų [M+H]+ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 791.1 g/mol |
|---|---|
| XLogP3 | -2.1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 11 |
| Exact Mass | 790.8698 g/mol |
| Monoisotopic Mass | 790.8698 g/mol |
| Topological Polar Surface Area | 169 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 647 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
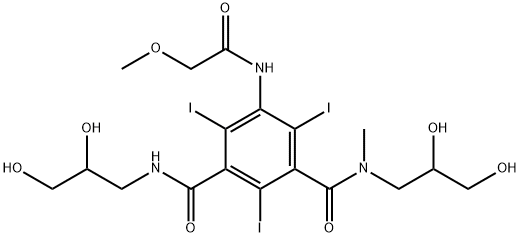
Iopromide is a dicarboxylic acid diamide that consists of N-methylisophthalamide bearing three iodo substituents at positions 2, 4 and 6, a methoxyacetyl substituent at position 5 and two 2,3-dihydroxypropyl groups attached to the amide nitrogens. A water soluble x-ray contrast agent for intravascular administration. It has a role as a radioopaque medium, a nephrotoxic agent, a xenobiotic and an environmental contaminant. It is an organoiodine compound and a dicarboxylic acid diamide. It is functionally related to an isophthalamide and a glycerol.