62883-00-5
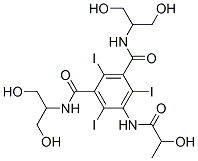
Product Name:
N1,N3-Bis(1,3-dihydroxypropan-2-yl)-5-(2-hydroxypropanamido)-2,4,6-triiodoisophthalamide
Formula:
C17H22I3N3O8
Synonyms:
(S)-N,N′-Bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo-5-lactamidoisophthalamide;Iopamidol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | White crystalline powder |
|---|---|
| Odor | Odorless |
| Melting Point | Decomposes at about 300 °C without melting |
| Solubility | Very soluble in water |
| LogP | log Kow = -2.42 |
| Optical Rotation | Specific optical rotation: -20.1 deg at 20 °C/D (c = 10 in water) |
| Decomposition | When heated to decomposition it emits very toxic fumes of /iodine and oxides of nitrogen/. |
| Ionization Efficiency | Positive |
| Dissociation Constants | pKa = 10.70 (25 °C) |
| Collision Cross Section | 205.65 Ų [M-H]- 219.24 Ų [M+H]+ |
| Other Experimental Properties | Physical properties of iopamidol in solution with hydrochloric acid and/or sodium hydroxide. Table: Percentage Iopamidol in Solution [Table#8056] |
COMPUTED DESCRIPTORS
| Molecular Weight | 777.1 g/mol |
|---|---|
| XLogP3 | -2.4 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 10 |
| Exact Mass | 776.8541 g/mol |
| Monoisotopic Mass | 776.8541 g/mol |
| Topological Polar Surface Area | 188 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 583 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Iopamidol is a benzenedicarboxamide compound having N-substituted carbamoyl groups at the 1- and 3-positions, iodo substituents at the 2-, 4- and 6-positions and a (2S)-2-hydroxypropanamido group at the 5-position. It has a role as a radioopaque medium, an environmental contaminant and a xenobiotic. It is a benzenedicarboxamide, an organoiodine compound and a pentol.
