CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless or white odorless hygroscopic solid; [Merck Index] Colorless odorless hygroscopic powder; [MSDSonline] |
|---|---|
| Color/Form | Colorless powder |
| Odor | Odorless |
| Melting Point | 130-135 °C (with decomp) |
| Solubility | Solubility: very soluble in water; soluble in dimethylformamide, dimethyl sulphoxide, methanol; sparingly soluble in acetone, ethanol. |
| Vapor Pressure | Negligible at room temperature |
| Stability/Shelf Life | Stable at room temperature and in neutral or alkaline media, slightly unstable in acidic media. |
| Corrosivity | Non-corrosive to metals |
| Ionization Efficiency | Positive |
| Other Experimental Properties | Stable at room temperature, in neutral and alkaline solution, but slightly unstable under acidic conditions. |
| Chemical Classes | Pesticides -> Fungicides |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H227:Flammable liquids |
| Precautionary Statement Codes |
P403+P235:Store in a well-ventilated place. Keep cool. |
COMPUTED DESCRIPTORS
| Molecular Weight | 497.5 g/mol |
|---|---|
| XLogP3 | -6.3 |
| Hydrogen Bond Donor Count | 12 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 7 |
| Exact Mass | 497.21084017 g/mol |
| Monoisotopic Mass | 497.21084017 g/mol |
| Topological Polar Surface Area | 253 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 697 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 14 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
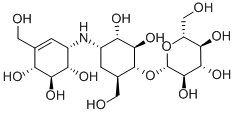
Validamycin A is a member of the class of validamycins that is (1R,2S,3S,4S,6R)-4-amino-6-(hydroxymethyl)cyclohexane-1,2,3-triol in which the hydroxy group at position 1 has been converted to its beta-D-glucoside and in which one of the hydrogens attached to the nitrogen is replaced by a (1R,4R,5R,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl group. It is the major validamycin produced by Streptomyces hygroscopicus. It has a role as an EC 2.4.1.231 [alpha,alpha-trehalose phosphorylase (configuration-retaining)] inhibitor, an EC 2.4.1.64 (alpha,alpha-trehalose phosphorylase) inhibitor, an EC 3.2.1.28 (alpha,alpha-trehalase) inhibitor and an antifungal agrochemical. It is a member of validamycins, a secondary amino compound, a polyol and an antibiotic fungicide. It is a conjugate base of a validamycin A(1+).