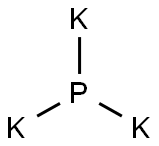
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Potassium phosphide is a white crystalline or powdered solid. When exposed to water it may react violently and start a fire. It is toxic by ingestion, inhalation and skin absorption. It is used to make other chemicals. |
|---|---|
| Color/Form | White crystalline or powdered solid |
| Other Experimental Properties | Decomposed by water or moisture in air, evolving toxic phosphine which often ignites |
COMPUTED DESCRIPTORS
| Molecular Weight | 150.285 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 149.8805315 g/mol |
| Monoisotopic Mass | 149.8805315 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 2 |
| Complexity | 3.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Potassium phosphide is a white crystalline or powdered solid. When exposed to water it may react violently and start a fire. It is toxic by ingestion, inhalation and skin absorption. It is used to make other chemicals.