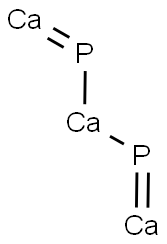
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Calcium phosphide appears as red-brown crystals to gray granular lumps. It reacts with water to form calcium hydroxide and phosphine, a flammable poisonous gas. Phosphine will normally ignite spontaneously in contact with air. If there is an excess of water this fire of phosphine will not normally ignite surrounding combustible material. |
|---|---|
| Color/Form | Red-brown crystalline powder or gray lumps |
| Odor | Musty, like acetylene |
| Boiling Point | Decomposes |
| Melting Point | 2910 °F (USCG, 1999) |
| Solubility | Insol in alcohol, ether; reacts with water |
| Density | 2.51 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | Stable if dry. |
| Decomposition | ... DECOMPOSED BY WATER TO PHOSPHINE, WHICH IS HIGHLY TOXIC ... |
| Odor Threshold | Odor Threshold Low: 0.13 [ppm] Odor Threshold High: 13.4 [ppm] Odor threshold = 1-100 mg/m3 |
| Other Experimental Properties | Decomposed by moist air or water, evolving spontaneously-flammable phosphine. |
| Chemical Classes | Metals -> Phosphides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H300:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P231+P232:Handle under inert gas. Protect from moisture. P370+P378:In case of fire: Use … for extinction. P422:Store contents under … |
COMPUTED DESCRIPTORS
| Molecular Weight | 182.18 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 181.8352965 g/mol |
| Monoisotopic Mass | 181.8352965 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 5 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Calcium phosphide appears as red-brown crystals to gray granular lumps. It reacts with water to form calcium hydroxide and phosphine, a flammable poisonous gas. Phosphine will normally ignite spontaneously in contact with air. If there is an excess of water this fire of phosphine will not normally ignite surrounding combustible material.