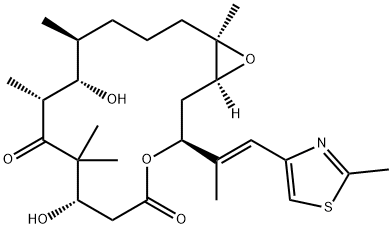
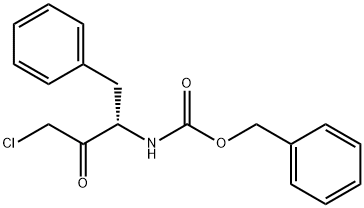
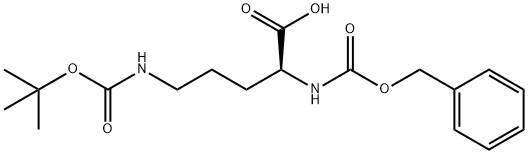
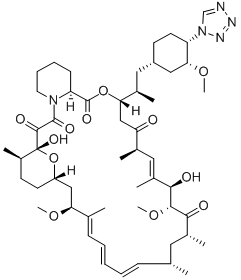
ZOTAROLIMUS
- CAS NO.:221877-54-9
- Empirical Formula: C52H79N5O12
- Molecular Weight: 966.21
- MDL number: MFCD09752954
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-21 16:49:52
What is ZOTAROLIMUS?
Description
Zotarolimus is a macrocyclic lactone immunosuppressant and a derivative of rapamycin . It binds to FKBP prolyl isomerase 1A (FKBP12; IC50 = 2.57 nM) and inhibits proliferation of human peripheral blood mononuclear cells (PBMCs), rat splenocytes, and human coronary artery smooth muscle cells (IC50s = 7, 1,337, and 0.8 nM, respectively). Zotarolimus has immunosuppressive activity in a one-way mixed lymphocyte reaction using human or rat lymphocytes (IC50s = 1.2 and 1,465 nM, respectively). It also reduces symptom severity in a rat model of experimental autoimmune encephalomyelitis (EAE; ED50 = 1.17 mg/kg per day) and delays cardiac allograft rejection in rats (ED50 = 3.71 mg/kg per day). Zotarolimus inhibits neointimal formation and reduces stenosis in pig coronary arteries when applied at 10 μg/mm to stainless steel balloon expandable stents with phosphorylcholine in a model of restenosis. Formulations containing zotarolimus have been used in drug-eluting stents in the prevention of restenosis following stent placement.
Chemical properties
Pale Yellow Solid
The Uses of ZOTAROLIMUS
Zotarolimus is a semi-synthetic macrocyclic lactone prepared from rapamycin by preparation of the 42-triflate ester, followed by displacement with tetrazole and purification of the two isomeric products. This structural change affords a less bioavailable product, a preferred profile for some applications. Like all tacrolimus analogues, zotarolimus binds to a receptor protein (FKBP12). The complex then binds to preventing it from interacting with target proteins. Zotarolimus is extensively cited in the literature with over 200 citations.
The Uses of ZOTAROLIMUS
A tetrazole-containing Rapamycin analog as immunomodulator and useful in the treatment of restenosis and immune and autoimmune diseases.
What are the applications of Application
Zotarolimus is a tetrazole-containing Rapamycin analog
Definition
ChEBI: Zotarolimus is a macrolide and a lactam.
Properties of ZOTAROLIMUS
| Melting point: | 100-105°C |
| Boiling point: | 1016.2±75.0 °C(Predicted) |
| Density | 1.25 |
| storage temp. | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| solubility | DMSO, Methanol (Slightly) |
| form | Solid |
| pka | 10.40±0.70(Predicted) |
| color | White to Pale Yellow |
Safety information for ZOTAROLIMUS
Computed Descriptors for ZOTAROLIMUS
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 Zotarolimus CAS 221877-54-9View Details
Zotarolimus CAS 221877-54-9View Details
221877-54-9 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8